LabAdviser/314/Preparation 314-307/Soft-matter: Difference between revisions
| (147 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
'''Feedback to this page''': '''[mailto:labadviser@nanolab.dtu.dk?Subject=Feed%20back%20from%20page%20http://labadviser.nanolab.dtu.dk/index.php/LabAdviser/314/Preparation_314-307/Soft-matter click here]''' | '''Feedback to this page''': '''[mailto:labadviser@nanolab.dtu.dk?Subject=Feed%20back%20from%20page%20http://labadviser.nanolab.dtu.dk/index.php/LabAdviser/314/Preparation_314-307/Soft-matter click here]''' | ||
[[Category:314]] | ''This section is written by DTU Nanolab internal if nothing else is stated.'' | ||
[[Category:314-Preparation]] | [[index.php?title=Category:314]] | ||
[[index.php?title=Category:314-Preparation]] | |||
= Plunge Freezer = | = Plunge Freezer = | ||
| Line 11: | Line 12: | ||
== Leica EM GP2 == | == Leica EM GP2 == | ||
Plunge freezing is a cryo-fixation method used to preserve samples in their | Plunge freezing is a cryo-fixation method used to preserve samples in their native state prior to cryo-electron microscopy. The EM GP2 plunge freezes fluid or extremely thin samples spread on an electron microscopy grid into liquid ethane and afterwards excess fluid is removed by automatic blotting. Prior to freezing, the sample is maintained in a temperature and humidity controlled environmental chamber which is adjustable between +4°C and +60°C and room humidity to 99 %. | ||
The main steps of this technique | The main steps of this technique is as follows:<br /> | ||
1. The sample is spread | 1. The sample is spread onto a glow discharged EM grid <br /> | ||
2. The liquid droplet is then blotted with filter paper until only a very thin film of fluid remains <br /> | 2. The liquid droplet is then blotted with filter paper until only a very thin film of fluid remains <br /> | ||
3. The grid is then rapidly plunged into a cryogen (usually liquid ethane) <br /> | 3. The grid is then rapidly plunged into a cryogen (usually liquid ethane) <br /> | ||
| Line 21: | Line 22: | ||
[https://labmanager.dtu.dk/view_binary.php?class=MiscDocument&id=15&name=Leica_EM_GP2_Operating_manual.pdf Operating manual] ''- requires login''<br /> | |||
[[File:Plunge Freezer (2).jpg|400px|left|thumb|Leica EM GP2 Location at DTU Nanolab Building | [[File:Plunge Freezer (2).jpg|400px|left|thumb|Leica EM GP2 Location at DTU Nanolab Building 307 Room 909B]]<br clear="all" /> | ||
For further information on the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information on the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
| Line 30: | Line 31: | ||
= High Pressure Freezer = | = High Pressure Freezer = | ||
== Leica EM ICE | == Leica EM ICE == | ||
High pressure freezing is key for the study of intricate changes in fine structure or cellular dynamics. | High pressure freezing is key for the study of intricate changes in fine structure or cellular dynamics. | ||
* Cryo-immobilize your aqueous samples under high pressure with a unique freezing principle and uncover secrets of the cellular process. | * Cryo-immobilize your aqueous samples under high pressure with a unique freezing principle and uncover secrets of the cellular process. | ||
| Line 46: | Line 47: | ||
[[File:HPF EM ICE.jpg|600px|left|thumb|Leica EM ICE Location at DTU Nanolab Building 307]]<br clear="all" /> | [[File:HPF EM ICE.jpg|600px|left|thumb|Leica EM ICE Location at DTU Nanolab Building 307 Room 909B]]<br clear="all" /> | ||
For further information on the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information on the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
| Line 52: | Line 53: | ||
= Freeze substitution = | = Freeze substitution = | ||
== Leica EM AFS2 and EM FSP | == Leica EM AFS2 and EM FSP == | ||
The Leica EM AFS2 performs freeze substitution and progressive lowering of temperature (PLT) techniques and allows low temperature embedding and polymerization of resins. The Leica EM FSP (freeze substitution processor), an automatic reagent handling system combined with the Leica EM AFS2, dispenses reagents for both freeze substitution and PLT applications. The LED illumination from within the chamber and the attached stereomicroscope for viewing and positioning of samples ensures ease of use. | The Leica EM AFS2 performs freeze substitution and progressive lowering of temperature (PLT) techniques and allows low temperature embedding and polymerization of resins. The Leica EM FSP (freeze substitution processor), an automatic reagent handling system combined with the Leica EM AFS2, dispenses reagents for both freeze substitution and PLT applications. The LED illumination from within the chamber and the attached stereomicroscope for viewing and positioning of samples ensures ease of use. | ||
[[File:Freeze-substitution EM AFS2.jpg|300px|left|thumb|Leica EM AFS2 Location at DTU Nanolab Building 307]] | [[File:Freeze-substitution EM AFS2.jpg|300px|left|thumb|Leica EM AFS2 Location at DTU Nanolab Building 307 Room 909B]] | ||
[[File:EM FSP.JPG|200px|left|thumb|Leica EM FSP Location at DTU Nanolab Building 307]]<br clear="all" /> | [[File:EM FSP.JPG|200px|left|thumb|Leica EM FSP Location at DTU Nanolab Building 307 Room 909B]]<br clear="all" /> | ||
For further information on the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information on the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
| Line 63: | Line 64: | ||
= Microtome = | = Microtome = | ||
== Leica EM UC7 Ultramicrotome== | == Leica EM UC7 Ultramicrotome== | ||
The Ultramicrotome Leica EM UC7 provides easy preparation of | The Ultramicrotome Leica EM UC7 provides easy sample preparation of ultrathin sections for TEM, SEM, AFM and LM application. It is suitable for highly skilled or absolute beginners. The Leica ultramicrotome can provide ultra thin sectioning of sample embedded in a resin/epoxy block with a feed range of 1 nm up to 15 µm. Glass or diamond knife can be used for the ultra sectioning of the samples. | ||
The Leica ultramicrotome | |||
| Line 84: | Line 71: | ||
1. Mount sample <br /> | 1. Mount sample <br /> | ||
2. Mount glass or diamond knife to knife holder <br /> | 2. Mount glass or diamond knife to knife holder <br /> | ||
3. Trimming | 3. Trimming or ultrasectioning of sample <br /> | ||
4. Fishing sections <br /> | 4. Fishing sections <br /> | ||
5. Transfer to an EM grid <br /> | 5. Transfer to an EM grid <br /> | ||
[https://labmanager.dtu.dk/view_binary.php?class=MiscDocument&id=15&name=EM_UC7_Operating_manual.pdf Operating manual] ''-requires login''<br /> | |||
[[File:Leica EM UC7.jpg|400px|left|thumb|Leica EM UC7 Location at DTU Nanolab Building 307 Room 906]]<br clear="all" /> | [[File:Leica EM UC7.jpg|400px|left|thumb|Leica EM UC7 Location at DTU Nanolab Building 307 Room 906]]<br clear="all" /> | ||
For further information about the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | |||
'''Requirement for training:''' Completion of the DTU Epoxy course and purchased of own diamond knife. For further information about the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | |||
<br clear="all" /> | <br clear="all" /> | ||
== Leica EM FC7 Cryo-Ultramicrotome == | == Leica EM FC7 Cryo-Ultramicrotome == | ||
Within minutes the Leica EM UC7 ultramicrotome can change to a cryo-ultramicrotome by mounting the cryo chamber EM FC7. | Within minutes the Leica EM UC7 ultramicrotome can be change to a cryo-ultramicrotome by mounting the cryo chamber EM FC7. Using the cryo-ultramicrotome, cryo-sections (-15° to -185°C) can be prepared for TEM, SEM, AFM and LM applications. | ||
| Line 104: | Line 91: | ||
1. Set up the cryo chamber EM FC7 to the EM UC7 apparatus <br /> | 1. Set up the cryo chamber EM FC7 to the EM UC7 apparatus <br /> | ||
2. Mount both the trim and sectioning diamond knives to the knive holder and set up in the cryo chamber <br /> | 2. Mount both the trim and sectioning diamond knives to the knive holder and set up in the cryo chamber <br /> | ||
3. | 3. Connect the pump tube between the liquid nitrogen Dewar and the ultramicrotome apparatus <br /> | ||
4. | 4. Fill up liquid nitrogen Dewar <br /> | ||
5. Let equipment to cool down for 1 hour <br /> | 5. Let equipment to cool down for 1 hour <br /> | ||
6. Meanwhile prepare samples <br /> | 6. Meanwhile prepare samples (Cut big samples to smaller pieces) <br /> | ||
7. | 7. Freeze sample in liquid nitrogen and mount sample <br /> | ||
8. Trimming and ultrasectioning of sample <br /> | 8. Trimming and ultrasectioning of sample <br /> | ||
9. Collecting sections and transfer to an EM grid <br /> | 9. Collecting sections and transfer to an EM grid <br /> | ||
[https://labmanager.dtu.dk/view_binary.php?class=MiscDocument&id=15&name=EM_FC7_Operating_manual.pdf Operating manual] ''- requires login''<br /> | |||
[[File:EM FC7.jpg|400px|left|thumb|Leica EM FC7 Location at DTU Nanolab Building 307 Room 906]]<br clear="all" /> | [[File:Leica EM FC7 Cryo-Ultramicrotome.jpg|400px|left|thumb|Leica EM FC7 Location at DTU Nanolab Building 307 Room 906]]<br clear="all" /> | ||
For further information about the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | |||
'''Requirement for training:''' Purchased of own diamond knife. For further information about the equipment usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | |||
<br clear="all" /> | <br clear="all" /> | ||
= KnifeMaker= | = KnifeMaker= | ||
== | == Leica EM KMR3 == | ||
At DTU Nanolab we offer the possibility to make glass knives using the | At DTU Nanolab we offer the possibility to make glass knives for ultramicrotomy applications using the Leica EM KMR3 Knifemaker. | ||
[[ | [[|300px|thumb|left|LKB Knifemaker Location at DTU Nanolab Building 307 Room 909B]]<br clear="all" /> | ||
For further information about the knifemaker usage and training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information about the knifemaker usage and training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
| Line 135: | Line 122: | ||
== Leica EM CPD300 == | == Leica EM CPD300 == | ||
The | The CPD allows for ideal preservation of specimen's microstructures during drying processes prior to SEM analysis. Since air drying could cause severe deformation and collapse of microstructure due to the effects of high surface tension to air, the water in the biological specimen is replaced with a suitable inert fluid (“transitional fluid”-CO2) which has a lower surface tension to air. This would thus reduce severe structural damages during drying processes. | ||
Because liquid CO2 is not sufficiently miscible with water, a third medium commonly Acetone or Ethanol is used as “intermediate fluid”. The specimen is first dehydrated through various concentrations of the intermediate fluid and thus completely replacing the water in the specimen. Afterwards, the “intermediate fluid” is replaced with the “transitional fluid” (CO2) which can then convert from liquid to gas phase without surface tension effects which distort morphology and ultra-structure. | |||
[[File:EM CPD300.jpg|300px|thumb|left|Leica EM CPD300 Location at DTU Nanolab Building | |||
[https://labmanager.dtu.dk/view_binary.php?class=MiscDocument&id=15&name=EM_CPD300_Operating_manual_small.pdf Operating manual] ''- requires login'' <br /> | |||
[[File:EM CPD300.jpg|300px|thumb|left|Leica EM CPD300 Location at DTU Nanolab Building 307 Room 903]]<br clear="all" /> | |||
For further information on the CPD usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information on the CPD usage or training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
<br clear="all" /> | <br clear="all" /> | ||
= | = Coater = | ||
== Leica EM ACE600 == | |||
[[ | [[|300px|thumb|left|Leica EM ACE600 Location at DTU Nanolab Building 307 Room 907]]<br clear="all" /> | ||
For further information | For further information about the knifemaker usage and training contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
<br clear="all" /> | <br clear="all" /> | ||
= Preparation equipment = | |||
== Mini tube rotator == | |||
The mini tube rotator can be used for rotating various sized laboratory tubes at several mixing angles at speeds ranging from 4 to 18 min–1. | |||
Specifications: | |||
* Variable speed with LCD display | * Variable speed with LCD display | ||
* Digital microprocessor control | * Digital microprocessor control | ||
| Line 165: | Line 159: | ||
* Cold room and incubator compatible | * Cold room and incubator compatible | ||
[[File:IMG_9636.jpg|400px|thumb|left|Mini tube rotator Location at DTU Nanolab Building 307 Room 903]]<br clear="all" /> | |||
[[File: | |||
For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
<br clear="all" /> | <br clear="all" /> | ||
= Centrifuge = | == Mini Centrifuge == | ||
The Eppendorf Mini Spin plus centrifuge is a desktop centrifuge | The Eppendorf Mini Spin plus centrifuge is a desktop centrifuge and has a user friendly digital display for time and speed. | ||
Specifications: | |||
* Max. capacity 12 x 1.5/2.0 mL | |||
* Max. RCF 14 100 g | |||
* Speed 800 - 14 500 rpm (100 rpm steps) | |||
* Acceleration time 13 s | |||
* Timer 15 s to 99 mins | |||
[https://labmanager.dtu.dk/view_binary.php?class=MiscDocument&id=15&name=Eppendorf_Mini_Spin_plus_centrifuge_Original_Instructions.pdf Instruction manual] ''- requires login'' <br /> | |||
[[File:Mini Spin centrifuge.jpg| | [[File:Mini Spin centrifuge.jpg|400px|thumb|left|Eppendorf Mini Spin centrifuge Location at DTU Nanolab Building 307 Room 903]]<br clear="all" /> | ||
For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
<br clear="all" /> | <br clear="all" /> | ||
= | == Magnetic stirrer == | ||
The myPlate magnetic stirrer is ideal for homogeneous mixing of chemicals. | |||
Specifications: | |||
*High chemical resistance | |||
*Can be used at 5 to 40 °C (80% relative humidity), in an incubator or cold room | |||
*Speed range 100 to 2500 (min-1) | |||
[[File: | [[File:myPlate magnetic stirrer.jpg|400px|thumb|left|myPlate magnetic stirrer Location at DTU Nanolab Building 307 Room 903]]<br clear="all" /> | ||
For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
<br clear="all" /> | <br clear="all" /> | ||
= Pulsing Vortex Mixer = | == Pulsing Vortex Mixer == | ||
VWR pulsing vortex mixer | The VWR pulsing vortex mixer provides ideal mixing of solutions or chemicals. | ||
Specifications according to the instruction manual: | |||
* Can be used at 4 to 40 °C (maximum 85% relative humidity, non condensing), in an incubator, CO₂ incubator or cold room | * Can be used at 4 to 40 °C (maximum 85% relative humidity, non condensing), in an incubator, CO₂ incubator or cold room | ||
* Pulsing model | * Pulsing model can reduce heat generation and ensures efficient mixing and disruption | ||
* | * Have timer from 1 s to 160 h | ||
[[File: | [https://labmanager.dtu.dk/view_binary.php?class=MiscDocument&id=15&name=VWR_pulsing_vortex_mixer_Instruction_Manual.pdf Instruction manual] ''- requires login'' <br /> | ||
[[File:IMG_9635.jpg|400px|thumb|left|VWR Pulsing Vortex Mixer Location at DTU Nanolab Building 307 Room 903]]<br clear="all" /> | |||
For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
<br clear="all" /> | <br clear="all" /> | ||
= | == Magnetic hotplate stirrer == | ||
VWR Professional hotplate stirrer is ideal for stirring chemicals with controlled high temperature. An external resistance thermometer (RTD) probe option is available for temperature control of the sample. | |||
Specifications: | |||
*Temperature range (°C) +5 to 400 | |||
*Speed range (min-1) 60 to 1600 | |||
*Excellent temperature uniformity with consistent stirring at all speeds | |||
*Separate digital displays for temperature, speed and time; show set and actual values. Display will show last used settings, even after power has been turned off | |||
*Cool touch, chemically resistant housing | |||
*Includes external RTD temperature probe kit | |||
[[File:Magnetic hotplate stirrer.jpeg|400px|thumb|left|VWR Magnetic hotplate stirrer Location at DTU Nanolab Building 314 Room 040]]<br clear="all" /> | |||
For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | |||
<br clear="all" /> | |||
== Hotplate == | |||
VWR Hotplate W10 is suitable for drying paraffin sections on microscope slides or for de-icing tools when doing cryo experiments. | |||
Specifications: | |||
*Electronic touch display | |||
*Sleek and compact | |||
*Ideal size for drying up to 40 microscope slides | |||
*Temperature range between ambient and +89 °C | |||
*Digital thermostat | |||
[https://labmanager.dtu.dk/view_binary.php?class=MiscDocument&id=15&name=VWR_Hotplate_W10_Instruction_Manual.pdf Instruction manual] ''- requires login'' <br /> | |||
[[File:VWR Hotplate W10.jpg|400px|thumb|left|VWR Hotplate W10 Location at DTU Nanolab Building 307 Room 903]]<br clear="all" /> | |||
For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | |||
<br clear="all" /> | |||
== Analytical balance PX224 == | |||
The Ohaus Pioneer PX224 offers high accuracy and repeatability weight measurements. | |||
Specifications: | |||
*Weighing capacity is 220 g | |||
*Readability is 0.1 mg | |||
*Stabilisation time is 4 s | |||
*A static removal bar (permanent static electric dissipative ABS) provides a convenient grounding location | |||
*Applications include basic weighing, part counting, percent weighing, dynamic weighing and density determination | |||
[[File:Ohaus Analytical Balance PX224.jpg|400px|thumb|left|Ohaus PX224 analytical balance Location at DTU Nanolab Building 307 Room 903]]<br clear="all" /> | |||
For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | |||
<br clear="all" /> | |||
== Midi 40 CO2 Incubator == | |||
The incubator is suitable for the incubation of cell culture and operates at temperatures ranging from 5°C above ambient temperature to 50°C ±0.2°C. | |||
Specifications: | |||
* Temperature range is from ambient +5° to 50°C ±0.2°C | |||
* Relative Humidity is <90% at 37°C | |||
* CO2 Sensor is TC Sensor | |||
* Capacity is 40L | |||
[[File:Midi 40 CO2 Incubator.jpg|400px|thumb|left|Midi 40 CO2 Incubator Location at DTU Nanolab Building 307 Room 903]]<br clear="all" /> | |||
For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | |||
<br clear="all" /> | |||
== Drying oven == | |||
The TS9026 drying oven is of 26L capacity and is used for epoxy embedding experiments. | |||
Specifications: | |||
* Temperature range is from 2°C to 250°C | |||
* Fan speed is 0 to 10 | |||
* Capacity is 26L | |||
[[File: | [[File:IMG 9634.jpg|400px|thumb|left|TS9026 Drying oven Location at DTU Nanolab Building 307 Room 903]]<br clear="all" /> | ||
For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | For further information on the equipment usage contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
<br clear="all" /> | <br clear="all" /> | ||
For '''TEM imaging''' of biological sample at room temperature or cryo conditions: <br clear="all" /> | = EM sample preparation workflow = | ||
For '''TEM imaging''' of biological and softmaterial sample at room temperature or cryo conditions: <br clear="all" /> | |||
[[File:EM sample prep for TEM.jpg|800px]] | [[File:EM sample prep for TEM.jpg|800px]] | ||
For '''SEM imaging''' of biological and softmaterial sample at room temperature or cryo conditions: <br clear="all" /> | |||
[[File:EM sample prep for SEM.jpg|700px]] | |||
For further information | For further information on EM sample preparation of biological and softmaterial samples contact mktracy@dtu.dk [https://www.dtu.dk/english/service/phonebook/person?id=141983&cpid=260636&tab=0]. | ||
<br clear="all" /> | <br clear="all" /> | ||
Latest revision as of 13:42, 13 November 2024
Feedback to this page: click here
This section is written by DTU Nanolab internal if nothing else is stated. index.php?title=Category:314 index.php?title=Category:314-Preparation
Plunge Freezer
Leica EM GP2
Plunge freezing is a cryo-fixation method used to preserve samples in their native state prior to cryo-electron microscopy. The EM GP2 plunge freezes fluid or extremely thin samples spread on an electron microscopy grid into liquid ethane and afterwards excess fluid is removed by automatic blotting. Prior to freezing, the sample is maintained in a temperature and humidity controlled environmental chamber which is adjustable between +4°C and +60°C and room humidity to 99 %.
The main steps of this technique is as follows:
1. The sample is spread onto a glow discharged EM grid
2. The liquid droplet is then blotted with filter paper until only a very thin film of fluid remains
3. The grid is then rapidly plunged into a cryogen (usually liquid ethane)
4. The grid is afterwards stored in a grid box submerged in liquid nitrogen until finally loaded into the cryo-electron microscope for imaging
Operating manual - requires login

For further information on the equipment usage or training contact mktracy@dtu.dk [1].
High Pressure Freezer
Leica EM ICE
High pressure freezing is key for the study of intricate changes in fine structure or cellular dynamics.
- Cryo-immobilize your aqueous samples under high pressure with a unique freezing principle and uncover secrets of the cellular process.
- Capture and resolve highly dynamic processes at the nanometer scale with millisecond precision.
The EM ICE solution, combining superior high pressure freezing with the possibility of light and electrical stimulation, is the platform for your discoveries.
Currently cryo-fixation is the only way to fix cellular constituents without introducing significant structural alterations.
The EM ICE solution uses a unique, alcohol-free freezing principle to allow a superior cryo-fixation of the specimen enabling better quality results to be obtained:
- No alcohol in the chamber leads to a faster pressure increase and immediate cooling of the specimen
- No alcohol residue on the carrier or the specimen
- Precisely define the timing for light and electrical stimulation

For further information on the equipment usage or training contact mktracy@dtu.dk [2].
Freeze substitution
Leica EM AFS2 and EM FSP
The Leica EM AFS2 performs freeze substitution and progressive lowering of temperature (PLT) techniques and allows low temperature embedding and polymerization of resins. The Leica EM FSP (freeze substitution processor), an automatic reagent handling system combined with the Leica EM AFS2, dispenses reagents for both freeze substitution and PLT applications. The LED illumination from within the chamber and the attached stereomicroscope for viewing and positioning of samples ensures ease of use.


For further information on the equipment usage or training contact mktracy@dtu.dk [3].
Microtome
Leica EM UC7 Ultramicrotome
The Ultramicrotome Leica EM UC7 provides easy sample preparation of ultrathin sections for TEM, SEM, AFM and LM application. It is suitable for highly skilled or absolute beginners. The Leica ultramicrotome can provide ultra thin sectioning of sample embedded in a resin/epoxy block with a feed range of 1 nm up to 15 µm. Glass or diamond knife can be used for the ultra sectioning of the samples.
The main steps of this technique proceed as follows:
1. Mount sample
2. Mount glass or diamond knife to knife holder
3. Trimming or ultrasectioning of sample
4. Fishing sections
5. Transfer to an EM grid
Operating manual -requires login

Requirement for training: Completion of the DTU Epoxy course and purchased of own diamond knife. For further information about the equipment usage or training contact mktracy@dtu.dk [4].
Leica EM FC7 Cryo-Ultramicrotome
Within minutes the Leica EM UC7 ultramicrotome can be change to a cryo-ultramicrotome by mounting the cryo chamber EM FC7. Using the cryo-ultramicrotome, cryo-sections (-15° to -185°C) can be prepared for TEM, SEM, AFM and LM applications.
The main steps of this technique proceed as follows:
1. Set up the cryo chamber EM FC7 to the EM UC7 apparatus
2. Mount both the trim and sectioning diamond knives to the knive holder and set up in the cryo chamber
3. Connect the pump tube between the liquid nitrogen Dewar and the ultramicrotome apparatus
4. Fill up liquid nitrogen Dewar
5. Let equipment to cool down for 1 hour
6. Meanwhile prepare samples (Cut big samples to smaller pieces)
7. Freeze sample in liquid nitrogen and mount sample
8. Trimming and ultrasectioning of sample
9. Collecting sections and transfer to an EM grid
Operating manual - requires login

Requirement for training: Purchased of own diamond knife. For further information about the equipment usage or training contact mktracy@dtu.dk [5].
KnifeMaker
Leica EM KMR3
At DTU Nanolab we offer the possibility to make glass knives for ultramicrotomy applications using the Leica EM KMR3 Knifemaker.
[[|300px|thumb|left|LKB Knifemaker Location at DTU Nanolab Building 307 Room 909B]]
For further information about the knifemaker usage and training contact mktracy@dtu.dk [6].
Critical point dryer
Leica EM CPD300
The CPD allows for ideal preservation of specimen's microstructures during drying processes prior to SEM analysis. Since air drying could cause severe deformation and collapse of microstructure due to the effects of high surface tension to air, the water in the biological specimen is replaced with a suitable inert fluid (“transitional fluid”-CO2) which has a lower surface tension to air. This would thus reduce severe structural damages during drying processes.
Because liquid CO2 is not sufficiently miscible with water, a third medium commonly Acetone or Ethanol is used as “intermediate fluid”. The specimen is first dehydrated through various concentrations of the intermediate fluid and thus completely replacing the water in the specimen. Afterwards, the “intermediate fluid” is replaced with the “transitional fluid” (CO2) which can then convert from liquid to gas phase without surface tension effects which distort morphology and ultra-structure.
Operating manual - requires login

For further information on the CPD usage or training contact mktracy@dtu.dk [7].
Coater
Leica EM ACE600
[[|300px|thumb|left|Leica EM ACE600 Location at DTU Nanolab Building 307 Room 907]]
For further information about the knifemaker usage and training contact mktracy@dtu.dk [8].
Preparation equipment
Mini tube rotator
The mini tube rotator can be used for rotating various sized laboratory tubes at several mixing angles at speeds ranging from 4 to 18 min–1.
Specifications:
- Variable speed with LCD display
- Digital microprocessor control
- An adjustable 0 – 90° mixing angle
- Interchangeable carousels for all common laboratory tubes
- Cold room and incubator compatible

For further information on the equipment usage contact mktracy@dtu.dk [9].
Mini Centrifuge
The Eppendorf Mini Spin plus centrifuge is a desktop centrifuge and has a user friendly digital display for time and speed.
Specifications:
- Max. capacity 12 x 1.5/2.0 mL
- Max. RCF 14 100 g
- Speed 800 - 14 500 rpm (100 rpm steps)
- Acceleration time 13 s
- Timer 15 s to 99 mins
Instruction manual - requires login

For further information on the equipment usage contact mktracy@dtu.dk [10].
Magnetic stirrer
The myPlate magnetic stirrer is ideal for homogeneous mixing of chemicals.
Specifications:
- High chemical resistance
- Can be used at 5 to 40 °C (80% relative humidity), in an incubator or cold room
- Speed range 100 to 2500 (min-1)

For further information on the equipment usage contact mktracy@dtu.dk [11].
Pulsing Vortex Mixer
The VWR pulsing vortex mixer provides ideal mixing of solutions or chemicals.
Specifications according to the instruction manual:
- Can be used at 4 to 40 °C (maximum 85% relative humidity, non condensing), in an incubator, CO₂ incubator or cold room
- Pulsing model can reduce heat generation and ensures efficient mixing and disruption
- Have timer from 1 s to 160 h
Instruction manual - requires login

For further information on the equipment usage contact mktracy@dtu.dk [12].
Magnetic hotplate stirrer
VWR Professional hotplate stirrer is ideal for stirring chemicals with controlled high temperature. An external resistance thermometer (RTD) probe option is available for temperature control of the sample.
Specifications:
- Temperature range (°C) +5 to 400
- Speed range (min-1) 60 to 1600
- Excellent temperature uniformity with consistent stirring at all speeds
- Separate digital displays for temperature, speed and time; show set and actual values. Display will show last used settings, even after power has been turned off
- Cool touch, chemically resistant housing
- Includes external RTD temperature probe kit

For further information on the equipment usage contact mktracy@dtu.dk [13].
Hotplate
VWR Hotplate W10 is suitable for drying paraffin sections on microscope slides or for de-icing tools when doing cryo experiments.
Specifications:
- Electronic touch display
- Sleek and compact
- Ideal size for drying up to 40 microscope slides
- Temperature range between ambient and +89 °C
- Digital thermostat
Instruction manual - requires login

For further information on the equipment usage contact mktracy@dtu.dk [14].
Analytical balance PX224
The Ohaus Pioneer PX224 offers high accuracy and repeatability weight measurements.
Specifications:
- Weighing capacity is 220 g
- Readability is 0.1 mg
- Stabilisation time is 4 s
- A static removal bar (permanent static electric dissipative ABS) provides a convenient grounding location
- Applications include basic weighing, part counting, percent weighing, dynamic weighing and density determination

For further information on the equipment usage contact mktracy@dtu.dk [15].
Midi 40 CO2 Incubator
The incubator is suitable for the incubation of cell culture and operates at temperatures ranging from 5°C above ambient temperature to 50°C ±0.2°C.
Specifications:
- Temperature range is from ambient +5° to 50°C ±0.2°C
- Relative Humidity is <90% at 37°C
- CO2 Sensor is TC Sensor
- Capacity is 40L

For further information on the equipment usage contact mktracy@dtu.dk [16].
Drying oven
The TS9026 drying oven is of 26L capacity and is used for epoxy embedding experiments.
Specifications:
- Temperature range is from 2°C to 250°C
- Fan speed is 0 to 10
- Capacity is 26L

For further information on the equipment usage contact mktracy@dtu.dk [17].
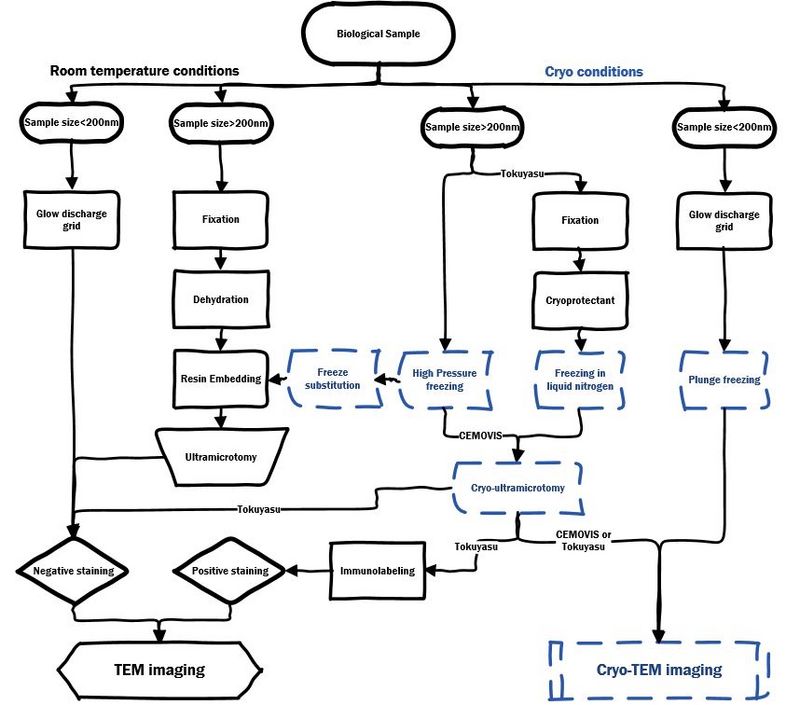
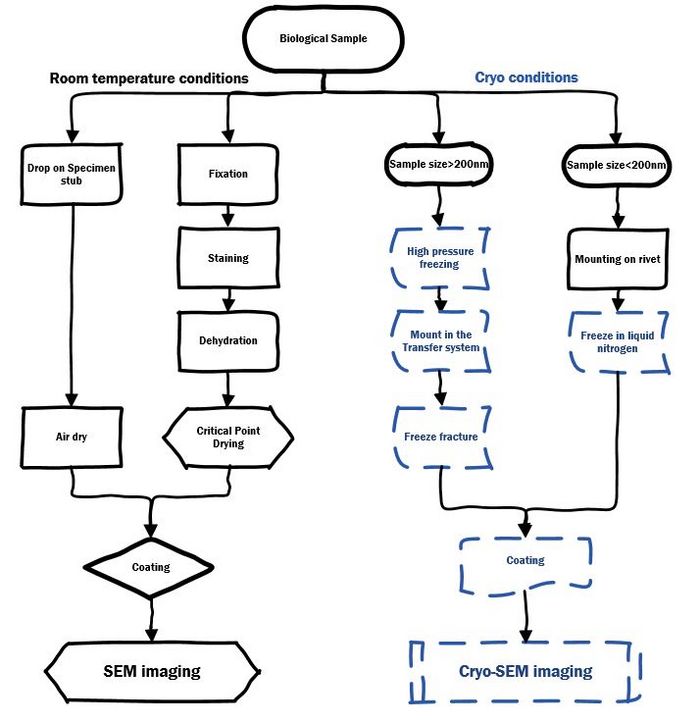
EM sample preparation workflow
For TEM imaging of biological and softmaterial sample at room temperature or cryo conditions:
For SEM imaging of biological and softmaterial sample at room temperature or cryo conditions:
For further information on EM sample preparation of biological and softmaterial samples contact mktracy@dtu.dk [18].
