Specific Process Knowledge/Characterization/XPS/XPS technique
Feedback to this page: click here
XPS technique
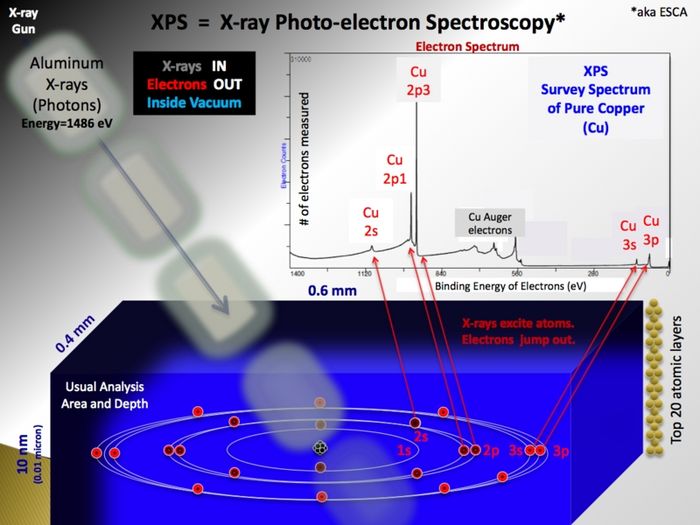
XPS is a surface sensitive and non destructive technique used for analysis of the elemental composition of a sample. It relies on the photoelectric effect; X-ray photons irradiated onto a sample will cause electrons bound in the sample atoms to become free electrons inside the the sample. The kinetic energy of these photoelectrons depend on the energy of the X-ray photon and their original binding energy. Escaping into the vacuum and counted in the electron spectrometer as function of their kinetic energy, the photoelectrons make a spectrum if represented in terms of numbers as function of their binding energy.
There are two reasons why the technique is extremely useful:
- Chemical sensitivity: The kinetic energy of the photoelectrons may be determined with such high accuracy (for instance, the resolution of the spectrometer is roughly 1 eV compared to the ~130 eV resolution of the spectrometer in an EDX X-ray detector ) that the small changes in binding energy of the sample electrons caused by the binding to other atoms may be detected. It is therefore possible to obtain information about the chemical environment of the elements - for instance in terms of:
- Oxidation state
- Double or triple bonds
- Surface states
- Surface sensitivity: The inelastic mean free path of the photoelectrons is very short - as a result, photoelectrons from depths larger than some 10 monolayers will not contribute to the peaks as they have lost part of their kinetic energy. This makes XPS extremely surface sensitive.
The basic principle is shown below (the image is taken from Wikipedia).
The analysis relies on the sequence:
- The X-ray source
- In the X-ray gun, electrons are extracted from a filament and accelerated by a high voltage onto an aluminium anode. Here, much like the primary beam in an SEM, X-rays with the characteristic energy of aluminium (1486.7 eV) are generated. Emitted isotropically, some X-rays hit a quartz crystal that act as monochromator as the X-rays diffract on the crystal planes according to the Bragg equation. If curved, the crystal will also focus the beam of X-rays and in this way enable us to use X-ray spots (in the shape of ellipses) of different sizes - ranging from 400 µm to 40 µm. The smaller spots, however, only come at the price of a drastically lowered intensity - therefore it is generally advised only to use the default value of 400 µm unless strictly necessary for some reason.
- Generation of photoelectrons
- As the incoming and monochromatic X-rays (with energy Ephot) impinge on and travel through the sample, they react with electrons bound to atoms in the sample with a certain binding energy (Ebind). The result is free electron inside sample with a kinetic energy (Ekin=Ephot-Ebind) that is characteristic of the atomic level it originated from. As the binding energy depends on the small energy changes in the electronic states induced by the formation of chemical bonds, the electron also carries information about the chemical state.
- Transport towards the vacuum
- The inelastic mean free path of the photoelectrons travelling inside a solid is very short in this energy range. The information carried by the photoelectrons will therefore quickly degrade as they collide inelastically and loose part of their energy. This means that only photoelectrons originating from the ~10 topmost atomic layers of the sample contribute to the signal - in this way making XPS extremely surface sensitive. The tail of former photoelectrons with non-specific energy is called the background. From inside the sample the electrons must pass the socalled work function in order to escape into the vacuum.
- Detection in the analyzer
- In the analyzer the electrons are separated according to the kinetic energy by two concentric spheres held at some potential. By playing on the acceptance angle of the entrance/exit slits of the analyzer one can tune the energy resolution with the parameter called the pass energy. Subsequently detected by the spectrometer, the number of electrons may be plotted as a function of energy thus making up an XPS spectrum.
More information on XPS
There is a vast number of textbooks on XPS and the internet/Youtube has plenty information as well. For the training sessions we have chosen a document entitled 'Electron Spectroscopy of surfaces' by Peter S. Deimel and Francesco Allegretti from the Technical University of Munich. It relatively short, yet thorough and takes you through theory and hardware. Skip sections 6-8. Click here to go the page with external material.
For the practical part of the training, some videos have been made. They can only be accessed using this link: