Specific Process Knowledge/Etch/Aluminum Oxide/Al2O3 Etch using HF: Difference between revisions
| Line 20: | Line 20: | ||
</ul> | </ul> | ||
Be aware of that the 5% HF etches quite fast | Be aware of that the 5% HF etches quite fast. Actually, so fast that it can be tricky to control. Taking the sample out of the solution and placing it in a bigger water container to rinse, resulting a time delay due to the movement and the etch continues. It means the handling things around the fumehood can be a source of errors in this case. | ||
''Evgeniy Shkondin, DTU Nanolab, June 2019'' | ''Evgeniy Shkondin, DTU Nanolab, June 2019'' | ||
Revision as of 10:58, 26 August 2019
Feedback to this page: click here
A wet chemical etch of Al2O3 can be done with HF. The etch rate depends on the HF concentration.
Experiment and results
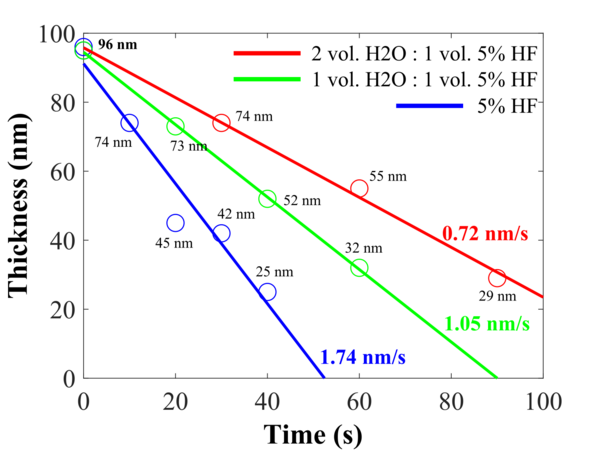
Si samples with about 100 nm of ALD deposited Al2O3 (1000 cycles at 300oC) has been etched in different HF concentrations. After the etching, the thickness of the Al2O3 layer has been measured, and the thickness as function of time has been plotted as shown in the graph below.
-
Different etching rates for different HF concentrations.
The etch rates:
- 5% HF: 1.74 nm/s
- 1vol. H2O : 1vol. 5% HF 1.05 nm/s
- 2vol. H2O : 1vol. 5% HF 0.72 nm/s
Be aware of that the 5% HF etches quite fast. Actually, so fast that it can be tricky to control. Taking the sample out of the solution and placing it in a bigger water container to rinse, resulting a time delay due to the movement and the etch continues. It means the handling things around the fumehood can be a source of errors in this case.
Evgeniy Shkondin, DTU Nanolab, June 2019