Specific Process Knowledge/Characterization/XPS/XPS elemental composition: Difference between revisions
| Line 7: | Line 7: | ||
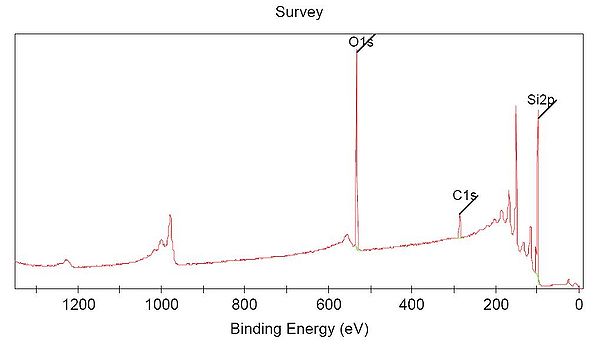
[[File:Overview spectra Labadvisor.JPG|600px|XPS spectrum of a sample consisting of the elements silicon, oxygen and carbon. ]] | [[File:Overview spectra Labadvisor.JPG|600px|XPS spectrum of a sample consisting of the elements silicon, oxygen and carbon. ]] | ||
With a unique set of electronic states, each element has its own "finger-print" in the XPS spectrum. Each electronic state is occupied by electrons with a specific binding energy. In the spectrum above, the dominant peaks of carbon, oxygen and silicon are labelled. More peaks are seen in this socalled survey spectrum but they are all associated to these three elements. | With a unique set of electronic states, each element has its own "finger-print" in the XPS spectrum. Each electronic state is occupied by electrons with a specific binding energy. In the spectrum above, the dominant peaks of carbon, oxygen and silicon are labelled. More peaks are seen in this socalled survey spectrum but they are all associated to these three elements. All elements have peaks within the wide energy range (usually 0 - 1350 eV binding energy) of the survey spectrum - hence any element that is present in the sample within its detection limit (roughly 1 % depending on element and acquisition setup) will show up in the survey spectrum as peaks of intensities that are determined by its concentration. | ||
show the | |||
In the table below, one can see the set | In the table below, one can see the set | ||
Revision as of 12:12, 14 May 2018
Feedback to this page: click here
Elemental composition analysis
With a unique set of electronic states, each element has its own "finger-print" in the XPS spectrum. Each electronic state is occupied by electrons with a specific binding energy. In the spectrum above, the dominant peaks of carbon, oxygen and silicon are labelled. More peaks are seen in this socalled survey spectrum but they are all associated to these three elements. All elements have peaks within the wide energy range (usually 0 - 1350 eV binding energy) of the survey spectrum - hence any element that is present in the sample within its detection limit (roughly 1 % depending on element and acquisition setup) will show up in the survey spectrum as peaks of intensities that are determined by its concentration. show the
In the table below, one can see the set
in atoms are different for all elements, and when measuring a photoelectron spectrum over a wide range of energies, the main line from each element will be placed at a specific energy in the spectrum.
Here is shown a spectrum measured over the energy range 0-1350 eV, and characteristic lines from three elements (C,O and Si) are seen and indicated in the spectrum.
The instrument program can use this information to give an estimate of the sample composition, giving the atomic percentage of the different elements.
Table with approximate binding energies
The table below has been adapted from [1].
| Element | K 1s | L1 2s | L2 2p1/2 | L3 2p3/2 | M1 3s | M2 3p1/2 | M3 3p3/2 | M4 3d3/2 | M5 3d5/2 | N1 4s | N2 4p1/2 | N3 4p3/2 | N5 4d5/2 | N6 4f5/2 | N7 4f7/2 | O1 5s | O2 5p1/2 | O3 5p3/2 | O4 5d3/2 | O5 5d5/2 | P1 6s | P2 6p1/2 | P3 6p3/2 |
| 1 H | 13,6 | ||||||||||||||||||||||
| 2 He | 24,6 | ||||||||||||||||||||||
| 3 Li | 54,7 | ||||||||||||||||||||||
| 4 Be | 111,5 | ||||||||||||||||||||||
| 5 B | 188 | ||||||||||||||||||||||
| 6 C | 284,2 | ||||||||||||||||||||||
| 7 N | 409,9 | 37,3 | |||||||||||||||||||||
| 8 O | 543,1 | 41,6 | |||||||||||||||||||||
| 9 F | 696,7 | ||||||||||||||||||||||
| 10 Ne | 870,2 | 48,5 | 21,7 | 21,6 | |||||||||||||||||||
| 11 Na | 1070,8 | 63,5 | 30,65 | 30,81 | |||||||||||||||||||
| 12 Mg | 1303 | 88,7 | 49,78 | 49,5 | |||||||||||||||||||
| 13 Al | 1559,6 | 117,8 | 72,95 | 72,55 | |||||||||||||||||||
| 14 Si | 1839 | 149,7 | 99,82 | 99,42 | |||||||||||||||||||
| 15 P | 2145,5 | 189 | 136 | 135 | |||||||||||||||||||
| 16 S | 2472 | 230,9 | 163,6 | 162,5 | |||||||||||||||||||
| 17 Cl | 2822,4 | 270 | 202 | 200 | |||||||||||||||||||
| 18 Ar | 3205,9 | 326,3 | 250,6 | 248,4 | 29,3 | 15,9 | 15,7 | ||||||||||||||||
| 19 K | 3608,4 | 378,6 | 297,3 | 294,6 | 34,8 | 18,3 | 18,3 | ||||||||||||||||
| 20 Ca | 4038,5 | 438,4 | 349,7 | 346,2 | 44,3 | 25,4 | 25,4 | ||||||||||||||||
| 21 Sc | 4492 | 498 | 403,6 | 398,7 | 51,1 | 28,3 | 28,3 | ||||||||||||||||
| 22 Ti | 4966 | 560,9 | 460,2 | 453,8 | 58,7 | 32,6 | 32,6 | ||||||||||||||||
| 23 V | 5465 | 626,7 | 519,8 | 512,1 | 66,3 | 37,2 | 37,2 | ||||||||||||||||
| 24 Cr | 5989 | 696 | 583,8 | 574,1 | 74,1 | 42,2 | 42,2 | ||||||||||||||||
| 25 Mn | 6539 | 769,1 | 649,9 | 638,7 | 82,3 | 47,2 | 47,2 | ||||||||||||||||
| 26 Fe | 7112 | 844,6 | 719,9 | 706,8 | 91,3 | 52,7 | 52,7 | ||||||||||||||||
| 27 Co | 7709 | 925,1 | 793,2 | 778,1 | 101 | 58,9 | 59,9 | ||||||||||||||||
| 28 Ni | 8333 | 1008,6 | 870 | 852,7 | 110,8 | 68 | 66,2 | ||||||||||||||||
| 29 Cu | 8979 | 1096,7 | 952,3 | 932,7 | 122,5 | 77,3 | 75,1 | ||||||||||||||||
| 30 Zn | 9659 | 1196,2 | 1044,9 | 1021,8 | 139,8 | 91,4 | 88,6 | 10,2 | 10,1 | ||||||||||||||
| 31 Ga | 10367 | 1299 | 1143,2 | 1116,4 | 159,5 | 103,5 | 100 | 18,7 | 18,7 | ||||||||||||||
| 32 Ge | 11103 | 1414,6 | 1248,1 | 1217 | 180,1 | 124,9 | 120,8 | 29,8 | 29,2 | ||||||||||||||
| 33 As | 11867 | 1527 | 1359,1 | 1323,6 | 204,7 | 146,2 | 141,2 | 41,7 | 41,7 | ||||||||||||||
| 34 Se | 12658 | 1652 | 1474,3 | 1433,9 | 229,6 | 166,5 | 160,7 | 55,5 | 54,6 | ||||||||||||||
| 35 Br | 13474 | 1782 | 1596 | 1550 | 257 | 189 | 182 | 70 | 69 | ||||||||||||||
| 36 Kr | 14326 | 1921 | 1730,9 | 1678,4 | 292,8 | 222,2 | 214,4 | 95 | 93,8 | 27,5 | 14,1 | 14,1 |
| Element | K 1s | L1 2s | L2 2p1/2 | L3 2p3/2 | M1 3s | M2 3p1/2 | M3 3p3/2 | M4 3d3/2 | M5 3d5/2 | N1 4s | N2 4p1/2 | N3 4p3/2 | N5 4d5/2 | N6 4f5/2 | N7 4f7/2 | O1 5s | O2 5p1/2 | O3 5p3/2 | O4 5d3/2 | O5 5d5/2 | P1 6s | P2 6p1/2 | P3 6p3/2 |
| 37 Rb | 15200 | 2065 | 1864 | 1804 | 326,7 | 248,7 | 239,1 | 113 | 112 | 30,5 | 16,3 | 15,3 | |||||||||||
| 38 Sr | 16105 | 2216 | 2007 | 1940 | 358,7 | 280,3 | 270 | 136 | 134,2 | 38,9 | 21,3 | 20,1 | |||||||||||
| 39 Y | 17038 | 2373 | 2156 | 2080 | 392 | 310,6 | 298,8 | 157,7 | 155,8 | 43,8 | 24,4 | 23,1 | |||||||||||
| 40 Zr | 17998 | 2532 | 2307 | 2223 | 430,3 | 343,5 | 329,8 | 181,1 | 178,8 | 50,6 | 28,5 | 27,1 | |||||||||||
| 41 Nb | 18986 | 2698 | 2465 | 2371 | 466,6 | 376,1 | 360,6 | 205 | 202,3 | 56,4 | 32,6 | 30,8 | |||||||||||
| 42 Mo | 20000 | 2866 | 2625 | 2520 | 506,3 | 411,6 | 394 | 231,1 | 227,9 | 63,2 | 37,6 | 35,5 | |||||||||||
| 43 Tc | 21044 | 3043 | 2793 | 2677 | 544 | 447,6 | 417,7 | 257,6 | 253,9 | 69,5 | 42,3 | 39,9 | |||||||||||
| 44 Ru | 22117 | 3224 | 2967 | 2838 | 586,1 | 483,5 | 461,4 | 284,2 | 280 | 75 | 46,3 | 43,2 | |||||||||||
| 45 Rh | 23220 | 3412 | 3146 | 3004 | 628,1 | 521,3 | 496,5 | 311,9 | 307,2 | 81,4 | 50,5 | 47,3 | |||||||||||
| 46 Pd | 24350 | 3604 | 3330 | 3173 | 671,6 | 559,9 | 532,3 | 340,5 | 335,2 | 87,1 | 55,7 | 50,9 | |||||||||||
| 47 Ag | 25514 | 3806 | 3524 | 3351 | 719 | 603,8 | 573 | 374 | 368,3 | 97 | 63,7 | 58,3 | |||||||||||
| 48 Cd | 26711 | 4018 | 3727 | 3538 | 772 | 652,6 | 618,4 | 411,9 | 405,2 | 109,8 | 63,9 | 63,9 | 11,7 | 10,7 | |||||||||
| 49 In | 27940 | 4238 | 3938 | 3730 | 827,2 | 703,2 | 665,3 | 451,4 | 443,9 | 122,9 | 73,5 | 73,5 | 17,7 | 16,9 | |||||||||
| 50 Sn | 29200 | 4465 | 4156 | 3929 | 884,7 | 756,5 | 714,6 | 493,2 | 484,9 | 137,1 | 83,6 | 83,6 | 24,9 | 23,9 | |||||||||
| 51 Sb | 30491 | 4698 | 4380 | 4132 | 946 | 812,7 | 766,4 | 537,5 | 528,2 | 153,2 | 95,6 | 95,6 | 33,3 | 32,1 | |||||||||
| 52 Te | 31814 | 4939 | 4612 | 4341 | 1006 | 870,8 | 820 | 583,4 | 573 | 169,4 | 103,3 | 103,3 | 41,9 | 40,4 | |||||||||
| 53 I | 33169 | 5188 | 4852 | 4557 | 1072 | 931 | 875 | 630,8 | 619,3 | 186 | 123 | 123 | 50,6 | 48,9 | |||||||||
| 54 Xe | 34561 | 5453 | 5107 | 4786 | 1148,7 | 1002,1 | 940,6 | 689 | 676,4 | 213,2 | 146,7 | 145,5 | 69,5 | 67,5 | — | — | 23,3 | 13,4 | 12,1 | ||||
| 55 Cs | 35985 | 5714 | 5359 | 5012 | 1211 | 1071 | 1003 | 740,5 | 726,6 | 232,3 | 172,4 | 161,3 | 79,8 | 77,5 | — | — | 22,7 | 14,2 | 12,1 | ||||
| 56 Ba | 37441 | 5989 | 5624 | 5247 | 1293 | 1137 | 1063 | 795,7 | 780,5 | 253,5 | 192 | 178,6 | 92,6 | 89,9 | — | — | 30,3 | 17 | 14,8 | ||||
| 57 La | 38925 | 6266 | 5891 | 5483 | 1362 | 1209 | 1128 | 853 | 836 | 274,7 | 205,8 | 196 | 105,3 | 102,5 | — | — | 34,3 | 19,3 | 16,8 | ||||
| 58 Ce | 40443 | 6549 | 6164 | 5723 | 1436 | 1274 | 1187 | 902,4 | 883,8 | 291 | 223,2 | 206,5 | 109 | — | 0,1 | 0,1 | 37,8 | 19,8 | 17 | ||||
| 59 Pr | 41991 | 6835 | 6440 | 5964 | 1511 | 1337 | 1242 | 948,3 | 928,8 | 304,5 | 236,3 | 217,6 | 115,1 | 115,1 | 2 | 2 | 37,4 | 22,3 | 22,3 | ||||
| 60 Nd | 43569 | 7126 | 6722 | 6208 | 1575 | 1403 | 1297 | 1003,3 | 980,4 | 319,2 | 243,3 | 224,6 | 120,5 | 120,5 | 1,5 | 1,5 | 37,5 | 21,1 | 21,1 | ||||
| 61 Pm | 45184 | 7428 | 7013 | 6459 | 1471 | 1357 | 1052 | 1027 | 242 | 242 | 120 | 120 | — | — | — | — | — | ||||||
| 62 Sm | 46834 | 7737 | 7312 | 6716 | 1723 | 1541 | 1420 | 1110,9 | 1083,4 | 347,2 | 265,6 | 247,4 | 129 | 129 | 5,2 | 5,2 | 37,4 | 21,3 | 21,3 | ||||
| 63 Eu | 48519 | 8052 | 7617 | 6977 | 1800 | 1614 | 1481 | 1158,6 | 1127,5 | 360 | 284 | 257 | 133 | 127,7 | 0 | 0 | 32 | 22 | 22 | ||||
| 64 Gd | 50239 | 8376 | 7930 | 7243 | 1881 | 1688 | 1544 | 1221,9 | 1189,6 | 378,6 | 286 | 271 | — | 142,6 | 8,6 | 8,6 | 36 | 28 | 21 | ||||
| 65 Tb | 51996 | 8708 | 8252 | 7514 | 1968 | 1768 | 1611 | 1276,9 | 1241,1 | 396 | 322,4 | 284,1 | 150,5 | 150,5 | 7,7 | 2,4 | 45,6 | 28,7 | 22,6 | ||||
| 66 Dy | 53789 | 9046 | 8581 | 7790 | 2047 | 1842 | 1676 | 1333 | 1292,6 | 414,2 | 333,5 | 293,2 | 153,6 | 153,6 | 8 | 4,3 | 49,9 | 26,3 | 26,3 | ||||
| 67 Ho | 55618 | 9394 | 8918 | 8071 | 2128 | 1923 | 1741 | 1392 | 1351 | 432,4 | 343,5 | 308,2 | 160 | 160 | 8,6 | 5,2 | 49,3 | 30,8 | 24,1 | ||||
| 68 Er | 57486 | 9751 | 9264 | 8358 | 2207 | 2006 | 1812 | 1453 | 1409 | 449,8 | 366,2 | 320,2 | 167,6 | 167,6 | — | 4,7 | 50,6 | 31,4 | 24,7 | ||||
| 69 Tm | 59390 | 10116 | 9617 | 8648 | 2307 | 2090 | 1885 | 1515 | 1468 | 470,9 | 385,9 | 332,6 | 175,5 | 175,5 | — | 4,6 | 54,7 | 31,8 | 25 | ||||
| 70 Yb | 61332 | 10486 | 9978 | 8944 | 2398 | 2173 | 1950 | 1576 | 1528 | 480,5 | 388,7 | 339,7 | 191,2 | 182,4 | 2,5 | 1,3 | 52 | 30,3 | 24,1 | ||||
| 71 Lu | 63314 | 10870 | 10349 | 9244 | 2491 | 2264 | 2024 | 1639 | 1589 | 506,8 | 412,4 | 359,2 | 206,1 | 196,3 | 8,9 | 7,5 | 57,3 | 33,6 | 26,7 | ||||
| 72 Hf | 65351 | 11271 | 10739 | 9561 | 2601 | 2365 | 2108 | 1716 | 1662 | 538 | 438,2 | 380,7 | 220 | 211,5 | 15,9 | 14,2 | 64,2 | 38 | 29,9 | ||||
| 73 Ta | 67416 | 11682 | 11136 | 9881 | 2708 | 2469 | 2194 | 1793 | 1735 | 563,4 | 463,4 | 400,9 | 237,9 | 226,4 | 23,5 | 21,6 | 69,7 | 42,2 | 32,7 | ||||
| 74 W | 69525 | 12100 | 11544 | 10207 | 2820 | 2575 | 2281 | 1872 | 1809 | 594,1 | 490,4 | 423,6 | 255,9 | 243,5 | 33,6 | 31,4 | 75,6 | 45,3 | 36,8 | ||||
| 75 Re | 71676 | 12527 | 11959 | 10535 | 2932 | 2682 | 2367 | 1949 | 1883 | 625,4 | 518,7 | 446,8 | 273,9 | 260,5 | 42,9 | 40,5 | 83 | 45,6 | 34,6 | ||||
| 76 Os | 73871 | 12968 | 12385 | 10871 | 3049 | 2792 | 2457 | 2031 | 1960 | 658,2 | 549,1 | 470,7 | 293,1 | 278,5 | 53,4 | 50,7 | 84 | 58 | 44,5 | ||||
| 77 Ir | 76111 | 13419 | 12824 | 11215 | 3174 | 2909 | 2551 | 2116 | 2040 | 691,1 | 577,8 | 495,8 | 311,9 | 296,3 | 63,8 | 60,8 | 95,2 | 63 | 48 | ||||
| 78 Pt | 78395 | 13880 | 13273 | 11564 | 3296 | 3027 | 2645 | 2202 | 2122 | 725,4 | 609,1 | 519,4 | 331,6 | 314,6 | 74,5 | 71,2 | 101,7 | 65,3 | 51,7 | ||||
| 79 Au | 80725 | 14353 | 13734 | 11919 | 3425 | 3148 | 2743 | 2291 | 2206 | 762,1 | 642,7 | 546,3 | 353,2 | 335,1 | 87,6 | 84 | 107,2 | 74,2 | 57,2 | ||||
| 80 Hg | 83102 | 14839 | 14209 | 12284 | 3562 | 3279 | 2847 | 2385 | 2295 | 802,2 | 680,2 | 576,6 | 378,2 | 358,8 | 104 | 99,9 | 127 | 83,1 | 64,5 | 9,6 | 7,8 | ||
| 81 Tl | 85530 | 15347 | 14698 | 12658 | 3704 | 3416 | 2957 | 2485 | 2389 | 846,2 | 720,5 | 609,5 | 405,7 | 385 | 122,2 | 117,8 | 136 | 94,6 | 73,5 | 14,7 | 12,5 | ||
| 82 Pb | 88005 | 15861 | 15200 | 13035 | 3851 | 3554 | 3066 | 2586 | 2484 | 891,8 | 761,9 | 643,5 | 434,3 | 412,2 | 141,7 | 136,9 | 147 | 106,4 | 83,3 | 20,7 | 18,1 | ||
| 83 Bi | 90524 | 16388 | 15711 | 13419 | 3999 | 3696 | 3177 | 2688 | 2580 | 939 | 805,2 | 678,8 | 464 | 440,1 | 162,3 | 157 | 159,3 | 119 | 92,6 | 26,9 | 23,8 | ||
| 84 Po | 93105 | 16939 | 16244 | 13814 | 4149 | 3854 | 3302 | 2798 | 2683 | 995 | 851 | 705 | 500 | 473 | 184 | 184 | 177 | 132 | 104 | 31 | 31 | ||
| 85 At | 95730 | 17493 | 16785 | 14214 | 4317 | 4008 | 3426 | 2909 | 2787 | 1042 | 886 | 740 | 533 | 507 | 210 | 210 | 195 | 148 | 115 | 40 | 40 | ||
| 86 Rn | 98404 | 18049 | 17337 | 14619 | 4482 | 4159 | 3538 | 3022 | 2892 | 1097 | 929 | 768 | 567 | 541 | 238 | 238 | 214 | 164 | 127 | 48 | 48 | 26 | |
| 87 Fr | 101137 | 18639 | 17907 | 15031 | 4652 | 4327 | 3663 | 3136 | 3000 | 1153 | 980 | 810 | 603 | 577 | 268 | 268 | 234 | 182 | 140 | 58 | 58 | 34 | 15 |
| 88 Ra | 103922 | 19237 | 18484 | 15444 | 4822 | 4490 | 3792 | 3248 | 3105 | 1208 | 1058 | 879 | 636 | 603 | 299 | 299 | 254 | 200 | 153 | 68 | 68 | 44 | 19 |
| 89 Ac | 106755 | 19840 | 19083 | 15871 | 5002 | 4656 | 3909 | 3370 | 3219 | 1269 | 1080 | 890 | 675 | 639 | 319 | 319 | 272 | 215 | 167 | 80 | 80 | — | — |
| 90 Th | 109651 | 20472 | 19693 | 16300 | 5182 | 4830 | 4046 | 3491 | 3332 | 1330 | 1168 | 966,4 | 712,1 | 675,2 | 342,4 | 333,1 | 290 | 229 | 182 | 92,5 | 85,4 | 41,4 | 24,5 |
| 91 Pa | 112601 | 21105 | 20314 | 16733 | 5367 | 5001 | 4174 | 3611 | 3442 | 1387 | 1224 | 1007 | 743 | 708 | 371 | 360 | 310 | 232 | 232 | 94 | 94 | — | — |
| 92 U | 115606 | 21757 | 20948 | 17166 | 5548 | 5182 | 4303 | 3728 | 3552 | 1439 | 1271 | 1043 | 778,3 | 736,2 | 388,2 | 377,4 | 321 | 257 | 192 | 102,8 | 94,2 | 43,9 | 26,8 |