Specific Process Knowledge/Characterization/XPS/Processing/Basics/3fitting: Difference between revisions
Appearance
| Line 4: | Line 4: | ||
== Fitting the oxygen O1s core level == | == Fitting the oxygen O1s core level == | ||
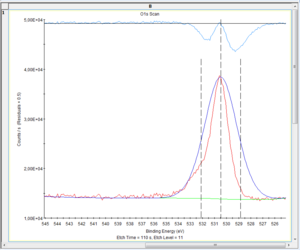
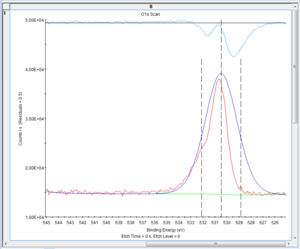
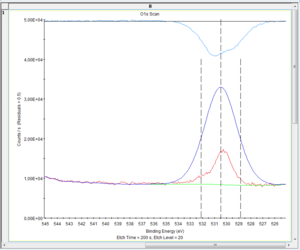
There is a few choices to make on the fitting of the O1s level. The level is an s-orbital and thus gives rise to a peak that is symmetric so any non-symmetric appearance must be caused several states of oxygen. The question is then to which states of oxygen they should be attributed. | There is a few choices to make on the fitting of the O1s level. The level is an s-orbital and thus gives rise to a peak that is symmetric so any non-symmetric appearance must be caused several states of oxygen. The question is then to which states of oxygen they should be attributed. Below are shown the spectra of levels 0 (surface), 11 and 20. | ||
[[Image:XPS-oxygenCapture 0022.PNG|300px| Level 0]] | [[Image:XPS-oxygenCapture 0022.PNG|300px| Level 0]] | ||
[[Image:XPS-oxygenCapture 0011.PNG|300px| Level 11]] | [[Image:XPS-oxygenCapture 0011.PNG|300px| Level 11]] | ||
[[Image:XPS-oxygenCapture 0033.PNG|300px| Level 20]] | [[Image:XPS-oxygenCapture 0033.PNG|300px| Level 20]] | ||
Revision as of 09:21, 28 June 2016
Feedback to this page: click here
Fitting the oxygen O1s core level
There is a few choices to make on the fitting of the O1s level. The level is an s-orbital and thus gives rise to a peak that is symmetric so any non-symmetric appearance must be caused several states of oxygen. The question is then to which states of oxygen they should be attributed. Below are shown the spectra of levels 0 (surface), 11 and 20.