Specific Process Knowledge/Characterization/XPS/XPS technique: Difference between revisions
| Line 15: | Line 15: | ||
; Generation of photoelectrons | ; Generation of photoelectrons | ||
: As the incoming and monochromatic X-rays (with energy E<sub>phot</sub>) impinge on and travel through the sample, they may react with electrons bound to atoms in the sample with a certain binding energy (E<sub>bind</sub>). The result is free electron inside sample with kinetic energy E<sub>kin</sub>=E<sub>phot</sub>-E<sub>bind</sub>. | : As the incoming and monochromatic X-rays (with energy E<sub>phot</sub>) impinge on and travel through the sample, they may react with electrons bound to atoms in the sample with a certain binding energy (E<sub>bind</sub>). The result is free electron inside sample with a kinetic energy (E<sub>kin</sub>=E<sub>phot</sub>-E<sub>bind</sub>) that is characteristic of the atomic level it originated from. The small energy changes in the electronic levels in the sample atoms induced by the formation of chemical bonds are also detectable by the XPS | ||
== X-ray Photoelectron Spectroscopy analysis (XPS) == | == X-ray Photoelectron Spectroscopy analysis (XPS) == | ||
Since the energy of the incoming photons is known, and the energy of the ejected electrons is measured, the binding energy of the electrons in the probed atoms can be determined. The binding energy of the electrons are element specific, and is therefore a "finger-print" of the atom. Hence, a measurement of the XPS spectrum gives information of which materials are present in the sample, and at which concentrations. | Since the energy of the incoming photons is known, and the energy of the ejected electrons is measured, the binding energy of the electrons in the probed atoms can be determined. The binding energy of the electrons are element specific, and is therefore a "finger-print" of the atom. Hence, a measurement of the XPS spectrum gives information of which materials are present in the sample, and at which concentrations. | ||
Revision as of 13:51, 31 August 2015
Feedback to this page: click here
XPS technique
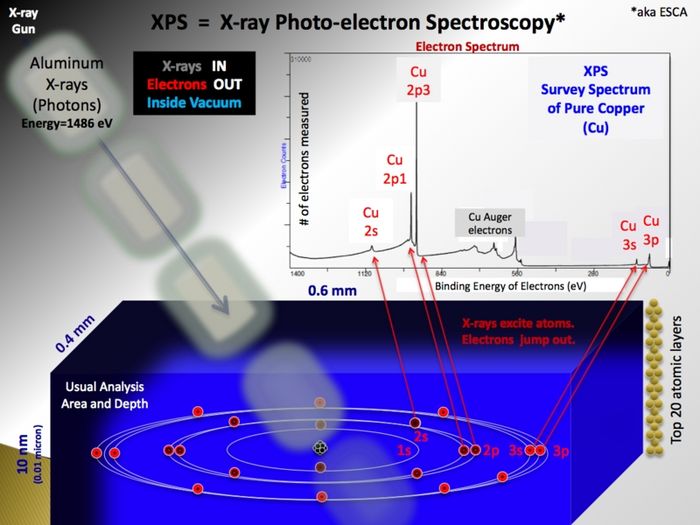
XPS is a surface sensitive and non destructive technique used for analysis of the elemental composition of a sample. The basic principle is shown below.
The analysis relies on the sequence:
- The X-ray source
- In the X-ray gun, electrons are extracted from a filament and accelerated by a high voltage onto an aluminium anode. Here, much like the primary beam in an SEM, X-rays with the characteristic energy of aluminium (1486.7 eV) are generated. Emitted isotropically, some X-rays hit a quartz crystal that act as monochromator as the X-rays diffract on the crystal planes according to the Bragg equation. If curved, the crystal will also focus the beam of X-rays and in this way enable us to use X-ray spots (in the shape of ellipses) of different sizes - ranging from 400 µm to 40 µm. The smaller spots, however, only come at the price of a drastically lowered intensity - therefore it is generally advised only to use the default value of 400 µm unless strictly necessary for some reason.
- Generation of photoelectrons
- As the incoming and monochromatic X-rays (with energy Ephot) impinge on and travel through the sample, they may react with electrons bound to atoms in the sample with a certain binding energy (Ebind). The result is free electron inside sample with a kinetic energy (Ekin=Ephot-Ebind) that is characteristic of the atomic level it originated from. The small energy changes in the electronic levels in the sample atoms induced by the formation of chemical bonds are also detectable by the XPS
X-ray Photoelectron Spectroscopy analysis (XPS)
Since the energy of the incoming photons is known, and the energy of the ejected electrons is measured, the binding energy of the electrons in the probed atoms can be determined. The binding energy of the electrons are element specific, and is therefore a "finger-print" of the atom. Hence, a measurement of the XPS spectrum gives information of which materials are present in the sample, and at which concentrations.
XPS is an excellent technique to probe the chemical state of atoms on a surface. The binding energy of lower lying atomic levels (for example 1s, 2s and 2p) are at a specific energy, but is slightly affected by the chemical environment of the probed atom. This is known as the chemical shift. By measuring the shift of the electron binding energies one can determined the chemical state of atoms. See an example on the page XPS-ThermoScientific.
Typical applications of XPS
The XPS can be used for different applications, for example:
- Do an elemental analysis of the outermost layer of your surface.
- Check the composition of a film at different depths.
- Check for a contaminations.
- It not as sensitive as the SIMS, but faster, so it can be an alternative if you are checking for a bit higher contamination levels (like 1 %)
- Do a analysis of the chemical state of atoms present on the surface.
- See what effect a surface treatment of your sample has on the surface chemistry.
- Check a polymer covered surface. Are for example (C=O), (C-OH) (C-C) groups present in the polymer after it been deposited on a surface.