Specific Process Knowledge/Characterization/Element analysis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[[image:EDX-scheme.jpg|300x300px|right|thumb|X-rays, with an energy that is characteristic of the atom they are emitted from, are created when the high energy electrons impinge on the sample. ]] | |||
You can make detailed analysis on the elemental composition and distribution in a sample with 3 instruments at Danchip. The [[Specific Process Knowledge/Characterization/SEM: Scanning Electron Microscopy/Leo|Leo SEM]] and [[Specific Process Knowledge/Characterization/SEM: Scanning Electron Microscopy/FEI|FEI SEM]] are both equipped with an X-ray detector that allows you to make elemental analysis by using the technique Energy Dispersive Analysis or EDX. The [[Specific Process Knowledge/Characterization/SIMS: Secondary Ion Mass Spectrometry|Atomika SIMS]] uses a technique called Secondary Ion Mass Spectrometry or SIMS. | You can make detailed analysis on the elemental composition and distribution in a sample with 3 instruments at Danchip. The [[Specific Process Knowledge/Characterization/SEM: Scanning Electron Microscopy/Leo|Leo SEM]] and [[Specific Process Knowledge/Characterization/SEM: Scanning Electron Microscopy/FEI|FEI SEM]] are both equipped with an X-ray detector that allows you to make elemental analysis by using the technique Energy Dispersive Analysis or EDX. The [[Specific Process Knowledge/Characterization/SIMS: Secondary Ion Mass Spectrometry|Atomika SIMS]] uses a technique called Secondary Ion Mass Spectrometry or SIMS. | ||
== Energy Dispersive X-ray analysis == | == Energy Dispersive X-ray analysis == | ||
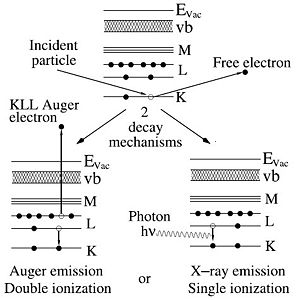
The technique of extracting information from the X-rays generated in a sample that is irradiated with electrons is called energy dispersive X-ray analysis or EDX. (Other acronyms are Energy Dispersive x-ray Spectroscopy, EDS, or Electron Probe Microanalysis, EPMA). The energetic electrons create core level vacancies as they collide with sample atoms electrons in a multiple scattering process. To decay from this excited state photons may be emitted. Their energy is determined by the difference in energy of the shells involved. Since atomic shells are unique for every element so will be the transitions between them. Thus, every element has its own characteristic X-ray spectrum that can be used to determine the elemental composition. | The technique of extracting information from the X-rays generated in a sample that is irradiated with electrons is called energy dispersive X-ray analysis or EDX. (Other acronyms are Energy Dispersive x-ray Spectroscopy, EDS, or Electron Probe Microanalysis, EPMA). The energetic electrons create core level vacancies as they collide with sample atoms electrons in a multiple scattering process. To decay from this excited state photons may be emitted. Their energy is determined by the difference in energy of the shells involved. Since atomic shells are unique for every element so will be the transitions between them. Thus, every element has its own characteristic X-ray spectrum that can be used to determine the elemental composition. | ||
Revision as of 16:12, 24 January 2008
You can make detailed analysis on the elemental composition and distribution in a sample with 3 instruments at Danchip. The Leo SEM and FEI SEM are both equipped with an X-ray detector that allows you to make elemental analysis by using the technique Energy Dispersive Analysis or EDX. The Atomika SIMS uses a technique called Secondary Ion Mass Spectrometry or SIMS.
Energy Dispersive X-ray analysis
The technique of extracting information from the X-rays generated in a sample that is irradiated with electrons is called energy dispersive X-ray analysis or EDX. (Other acronyms are Energy Dispersive x-ray Spectroscopy, EDS, or Electron Probe Microanalysis, EPMA). The energetic electrons create core level vacancies as they collide with sample atoms electrons in a multiple scattering process. To decay from this excited state photons may be emitted. Their energy is determined by the difference in energy of the shells involved. Since atomic shells are unique for every element so will be the transitions between them. Thus, every element has its own characteristic X-ray spectrum that can be used to determine the elemental composition.
Secondary Ion Mass Spectrometry
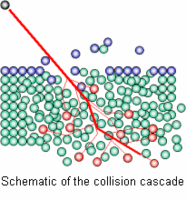
When a solid sample is sputtered by primary ions of few keV energy, a fraction of the particles emitted from the target is ionized. Secondary Ion Mass Spectrometry consists of analyzing these secondary ions with a mass spectrometer. Secondary ion emission by a solid surface under ion bombardment supplies information about the elemental, isotopic and molecular composition of its uppermost atomic layers.
SIMS is the most sensitive elemental and isotopic surface analysis technique. The secondary ion yields will vary greatly according to the chemical environment and the sputtering conditions (ion, energy, angle). This can add complexity to the quantitative aspect of the technique.
The SIMS technique provides a unique combination of extremely high sensitivity for all elements from Hydrogen to Uranium (detection limit down to ppb level for many elements), high lateral resolution imaging (down to 40 nm), and a very low background that allows high dynamic range (more than 5 decades). This technique is "destructive" by its nature (sputtering of material). It can be applied to any type of material (insulators, semiconductors, metals) that can stay under vacuum.
It allows molecular as well as elemental characterization of the first top monolayer in the static SIMS mode. It allows also the investigation of bulk composition or depth distribution of trace elements in the dynamic SIMS mode, with a depth resolution ranging from one to 20-30 nanometers.
This is why SIMS is one of the most widespread surface analysis techniques for advanced material research.
