Specific Process Knowledge/Thin film deposition/MVD: Difference between revisions
| Line 9: | Line 9: | ||
The MVD coatings are created as self-assembled monolayers on a surface when a molecular vapor of chemials is present. | The MVD coatings are created as self-assembled monolayers on a surface when a molecular vapor of chemials is present. | ||
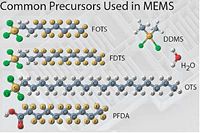
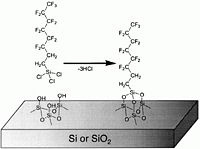
These chemicals, typically it is flourinated organosilanes, have a teflon-like tail consisting of -(CF<sub>2</sub>)<sub>x</sub>CF<sub>3</sub> and, in the other end, a reactive group -Si<sub>(teflon)</sub>Cl<sub>x</sub>. As shown in the figure below, the chlorine atoms react with -OH groups of the surface to form a chemical bond -Si(<sub>(teflon)</sub>)-O-Si<sub>(surface)</sub>- under elimination of HCL. This means that both Si and SiO<sub>2</sub> surfaces are coated. | These chemicals, typically it is flourinated organosilanes, have a teflon-like tail consisting of -(CF<sub>2</sub>)<sub>x</sub>CF<sub>3</sub> and, in the other end, a reactive group -Si<sub>(teflon)</sub>Cl<sub>x</sub>. As shown in the figure below, the chlorine atoms react with -OH groups of the surface to form a chemical bond -Si(<sub>(teflon)</sub>)-O-Si<sub>(surface)</sub>- under elimination of HCL. This means that both Si and SiO<sub>2</sub> surfaces are coated because of the native oxide on Si surfaces. | ||
<gallery caption="Some chemicals of the MVD and the surface reaction" widths="200px" heights="150px" perrow="2"> | <gallery caption="Some chemicals of the MVD and the surface reaction" widths="200px" heights="150px" perrow="2"> | ||
| Line 17: | Line 15: | ||
image:MVDsurfacereaction.jpg|The chemical reaction in which the Cl atoms of the precursors are eliminated under formation of HCl. | image:MVDsurfacereaction.jpg|The chemical reaction in which the Cl atoms of the precursors are eliminated under formation of HCl. | ||
</gallery> | </gallery> | ||
== The parameters of the MVD process == | |||
The most important parameters to control during the MVD process are: | |||
{| border="2" cellpadding="2" cellspacing="1" | {| border="2" cellpadding="2" cellspacing="1" | ||
Revision as of 16:25, 3 April 2008
The Molecular Vapor Deposition Tool

The Applied Microstructures MVD 100 system deposits molecular films on surfaces. These films serve a wide range of purposes ranging from antistiction coatings of nanoimprint lithography stamps to protecting MEMS structures. At Danchip the MVD is essential for nanoimprint lithography.
Processing on the MVD
The MVD coatings are created as self-assembled monolayers on a surface when a molecular vapor of chemials is present.
These chemicals, typically it is flourinated organosilanes, have a teflon-like tail consisting of -(CF2)xCF3 and, in the other end, a reactive group -Si(teflon)Clx. As shown in the figure below, the chlorine atoms react with -OH groups of the surface to form a chemical bond -Si((teflon))-O-Si(surface)- under elimination of HCL. This means that both Si and SiO2 surfaces are coated because of the native oxide on Si surfaces.
- Some chemicals of the MVD and the surface reaction
-
Different chemicals for the MVD.
-
The chemical reaction in which the Cl atoms of the precursors are eliminated under formation of HCl.
The parameters of the MVD process
The most important parameters to control during the MVD process are:
| O2 plasma | Flow | 200 sccm |
|---|---|---|
| Power | 250 Watts | |
| Time | 300 seconds | |
| Chemical # 1 (vapor order 1) | Name | FDTS |
| Line no. | 3 | |
| Cycles | 4 | |
| Pressure | 0.500 Torr | |
| Chemical # 2 (vapor order 2) | Name | Water |
| Line no. | 1 | |
| Cycles | 1 | |
| Pressure | 18 Torr | |
| Processing | Time | 900 seconds |
| Purge | Cycles | 5 |