Specific Process Knowledge/Characterization/XPS/XPS elemental composition: Difference between revisions
No edit summary |
|||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Feedback to this page''': '''[mailto:labadviser@ | '''Feedback to this page''': '''[mailto:labadviser@nanolab.dtu.dk?Subject=Feed%20back%20from%20page%20http://labadviser.nanolab.dtu.dk/index.php?title=Specific_Process_Knowledge/Characterization/XPS/XPS_elemental_composition click here]''' | ||
<!-- Page reviewed 9/8-2022 jmli --> | |||
<!--Checked for updates on 4/9-2025 - ok/jmli --> | |||
=Elemental composition analysis= | =Elemental composition analysis= | ||
{{Author-jmli1}} | |||
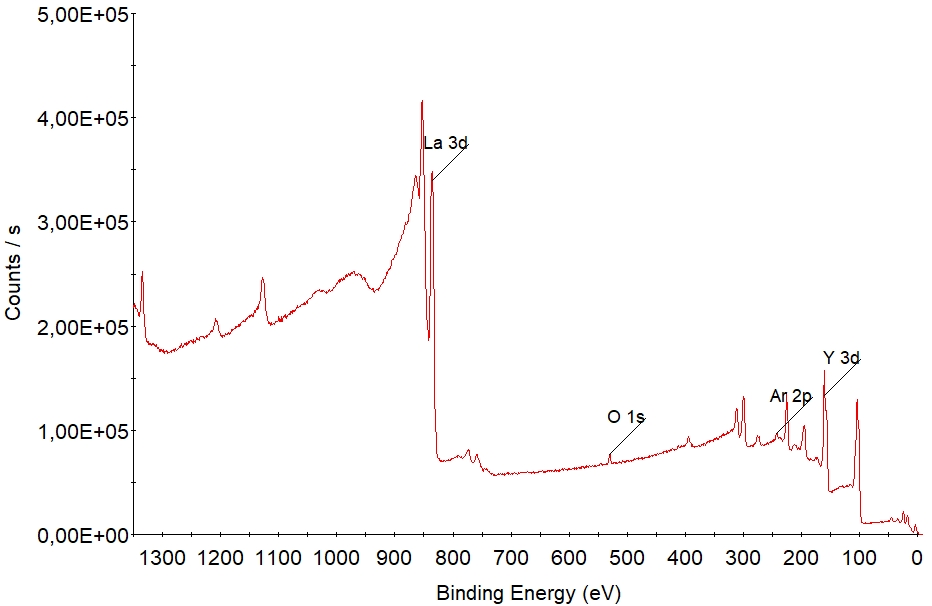
[[File: | [[File:XPS survey.jpg|XPS spectrum of a sample consisting of the elements silicon, oxygen and carbon. {{image1}}]] | ||
With a unique set of electronic states, each element has its own "finger-print" in the XPS spectrum. The electronic states in the atoms are occupied by electrons with a specific binding energy. In the spectrum above, the dominant peaks of carbon, oxygen and silicon are labelled. More peaks are seen in this socalled survey spectrum but they are all associated to these three elements. All elements have peaks within the wide energy range (usually 0 - 1350 eV binding energy) of the survey spectrum - hence any element that is present in the sample within its detection limit (roughly 1 % depending on element and acquisition setup) will show up in the survey spectrum as peaks of intensities that are determined by the respective concentrations. | With a unique set of electronic states, each element has its own "finger-print" in the XPS spectrum. The electronic states in the atoms are occupied by electrons with a specific binding energy. In the spectrum above, the dominant peaks of carbon, oxygen and silicon are labelled. More peaks are seen in this socalled survey spectrum but they are all associated to these three elements. All elements have peaks within the wide energy range (usually 0 - 1350 eV binding energy) of the survey spectrum - hence any element that is present in the sample within its detection limit (roughly 1 % depending on element and acquisition setup) will show up in the survey spectrum as peaks of intensities that are determined by the respective concentrations. | ||
| Line 14: | Line 17: | ||
== Table with approximate binding energies == | == Table with approximate binding energies == | ||
The table below has been adapted from | The table below has been adapted from the web archive at https://xdb.lbl.gov/Section1/Sec_1-1.html. | ||
=Hydrogen to copper= | =Hydrogen (1) to copper (29)= | ||
{| border="2" cellspacing="0" cellpadding="1" {{table}} | {| border="2" cellspacing="0" cellpadding="1" {{table}} | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''Orbital''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''1s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>3/2</sub>''' | ||
|- | |- | ||
| ''' 1 H'''||13,6|||||||||||| | | ''' 1 H'''||13,6|||||||||||| | ||
| Line 90: | Line 91: | ||
|} | |} | ||
= Zink to iodine = | = Zink (30) to iodine (53) = | ||
{| border="2" cellspacing="0" cellpadding="1" {{table}} | {| border="2" cellspacing="0" cellpadding="1" {{table}} | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''Orbital''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''1s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3d<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3d<sub>5/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4d<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4d<sub>5/2</sub>''' | ||
|- | |- | ||
| '''30 Zn'''||9659||1196,2||1044,9||1021,8||139,8||91,4||88,6||10,2||10,1|||||||||| | | '''30 Zn'''||9659||1196,2||1044,9||1021,8||139,8||91,4||88,6||10,2||10,1|||||||||| | ||
| Line 164: | Line 163: | ||
{| border="2" cellspacing="0" cellpadding="1" {{table}} | {| border="2" cellspacing="0" cellpadding="1" {{table}} | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''Orbital''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''1s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3d<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3d<sub>5/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4d<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4d<sub>5/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4f<sub>5/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4f<sub>7/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''5s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''5p<sub>1/2</sub> ''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''5p<sub>3/2</sub>''' | ||
|- | |- | ||
| '''54 Xe'''||34561||5453||5107||4786||1148,7||1002,1||940,6||689||676,4||213,2||146,7||145,5||69,5||67,5||—||—||23,3||13,4||12,1 | | '''54 Xe'''||34561||5453||5107||4786||1148,7||1002,1||940,6||689||676,4||213,2||146,7||145,5||69,5||67,5||—||—||23,3||13,4||12,1 | ||
| Line 244: | Line 241: | ||
{| border="2" cellspacing="0" cellpadding="1" {{table}} | {| border="2" cellspacing="0" cellpadding="1" {{table}} | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''Orbital''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''1s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3d<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3d<sub>5/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4d<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4d<sub>5/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4f<sub>5/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''4f<sub>7/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''5s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''5p<sub>1/2</sub> ''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''5p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''5d<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''5d<sub>5/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''6s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''6p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''6p<sub>3/2</sub>''' | ||
|- | |- | ||
| 80 Hg||83102||14839||14209||12284||3562||3279||2847||2385||2295||802,2||680,2||576,6||378,2||358,8||104||99,9||127||83,1||64,5||9,6||7,8|||||| | | '''80 Hg'''||83102||14839||14209||12284||3562||3279||2847||2385||2295||802,2||680,2||576,6||378,2||358,8||104||99,9||127||83,1||64,5||9,6||7,8|||||| | ||
|- | |- | ||
| 81 Tl||85530||15347||14698||12658||3704||3416||2957||2485||2389||846,2||720,5||609,5||405,7||385||122,2||117,8||136||94,6||73,5||14,7||12,5|||||| | | '''81 Tl'''||85530||15347||14698||12658||3704||3416||2957||2485||2389||846,2||720,5||609,5||405,7||385||122,2||117,8||136||94,6||73,5||14,7||12,5|||||| | ||
|- | |- | ||
| 82 Pb||88005||15861||15200||13035||3851||3554||3066||2586||2484||891,8||761,9||643,5||434,3||412,2||141,7||136,9||147||106,4||83,3||20,7||18,1|||||| | | '''82 Pb'''||88005||15861||15200||13035||3851||3554||3066||2586||2484||891,8||761,9||643,5||434,3||412,2||141,7||136,9||147||106,4||83,3||20,7||18,1|||||| | ||
|- | |- | ||
| 83 Bi||90524||16388||15711||13419||3999||3696||3177||2688||2580||939||805,2||678,8||464||440,1||162,3||157||159,3||119||92,6||26,9||23,8|||||| | | '''83 Bi'''||90524||16388||15711||13419||3999||3696||3177||2688||2580||939||805,2||678,8||464||440,1||162,3||157||159,3||119||92,6||26,9||23,8|||||| | ||
|- | |- | ||
| 84 Po||93105||16939||16244||13814||4149||3854||3302||2798||2683||995||851||705||500||473||184||184||177||132||104||31||31|||||| | | '''84 Po'''||93105||16939||16244||13814||4149||3854||3302||2798||2683||995||851||705||500||473||184||184||177||132||104||31||31|||||| | ||
|- | |- | ||
| 85 At||95730||17493||16785||14214||4317||4008||3426||2909||2787||1042||886||740||533||507||210||210||195||148||115||40||40|||||| | | '''85 At'''||95730||17493||16785||14214||4317||4008||3426||2909||2787||1042||886||740||533||507||210||210||195||148||115||40||40|||||| | ||
|- | |- | ||
| 86 Rn||98404||18049||17337||14619||4482||4159||3538||3022||2892||1097||929||768||567||541||238||238||214||164||127||48||48||26|||| | | '''86 Rn'''||98404||18049||17337||14619||4482||4159||3538||3022||2892||1097||929||768||567||541||238||238||214||164||127||48||48||26|||| | ||
|- | |- | ||
| 87 Fr||101137||18639||17907||15031||4652||4327||3663||3136||3000||1153||980||810||603||577||268||268||234||182||140||58||58||34||15||15 | | '''87 Fr'''||101137||18639||17907||15031||4652||4327||3663||3136||3000||1153||980||810||603||577||268||268||234||182||140||58||58||34||15||15 | ||
|- | |- | ||
| 88 Ra||103922||19237||18484||15444||4822||4490||3792||3248||3105||1208||1058||879||636||603||299||299||254||200||153||68||68||44||19|| 19 | | '''88 Ra'''||103922||19237||18484||15444||4822||4490||3792||3248||3105||1208||1058||879||636||603||299||299||254||200||153||68||68||44||19|| 19 | ||
|- | |- | ||
| 89 Ac||106755||19840||19083||15871||5002||4656||3909||3370||3219||1269||1080||890||675||639||319||319||272||215||167||80||80||—||—||- | | '''89 Ac'''||106755||19840||19083||15871||5002||4656||3909||3370||3219||1269||1080||890||675||639||319||319||272||215||167||80||80||—||—||- | ||
|- | |- | ||
| 90 Th||109651||20472||19693||16300||5182||4830||4046||3491||3332||1330||1168||966,4||712,1||675,2||342,4||333,1||290||229||182||92,5||85,4||41,4||24,5||16,6 | | '''90 Th'''||109651||20472||19693||16300||5182||4830||4046||3491||3332||1330||1168||966,4||712,1||675,2||342,4||333,1||290||229||182||92,5||85,4||41,4||24,5||16,6 | ||
|- | |- | ||
| 91 Pa||112601||21105||20314||16733||5367||5001||4174||3611||3442||1387||1224||1007||743||708||371||360||310||232||232||94||94||—||—||- | | '''91 Pa'''||112601||21105||20314||16733||5367||5001||4174||3611||3442||1387||1224||1007||743||708||371||360||310||232||232||94||94||—||—||- | ||
|- | |- | ||
| 92 U||115606||21757||20948||17166||5548||5182||4303||3728||3552||1439||1271||1043||778,3||736,2||388,2||377,4||321||257||192||102,8||94,2||43,9||26,8||16,8 | | '''92 U'''||115606||21757||20948||17166||5548||5182||4303||3728||3552||1439||1271||1043||778,3||736,2||388,2||377,4||321||257||192||102,8||94,2||43,9||26,8||16,8 | ||
|- | |- | ||
|} | |} | ||
Latest revision as of 09:35, 4 September 2025
Feedback to this page: click here
Elemental composition analysis
Unless otherwise stated, all content on this page was created by Jonas Michael-Lindhard, DTU Nanolab
With a unique set of electronic states, each element has its own "finger-print" in the XPS spectrum. The electronic states in the atoms are occupied by electrons with a specific binding energy. In the spectrum above, the dominant peaks of carbon, oxygen and silicon are labelled. More peaks are seen in this socalled survey spectrum but they are all associated to these three elements. All elements have peaks within the wide energy range (usually 0 - 1350 eV binding energy) of the survey spectrum - hence any element that is present in the sample within its detection limit (roughly 1 % depending on element and acquisition setup) will show up in the survey spectrum as peaks of intensities that are determined by the respective concentrations. As such, survey spectra are used to determined what elements are present in the sample.
For each element identified in the survey spectrum, one typically runs a spectrum at the dominant peak. These spectra have a much narrower energy ranges and therefore much smaller energy steps - this enables us to achieve much better energy resolution and hence to see the finer details of each element. These spectra, called high resolution spectra, contain the chemical information that is the strong selling point of the XPS technique.
Table with approximate binding energies
The table below has been adapted from the web archive at https://xdb.lbl.gov/Section1/Sec_1-1.html.
Hydrogen (1) to copper (29)
| Orbital | 1s | 2s | 2p1/2 | 2p3/2 | 3s | 3p1/2 | 3p3/2 |
| 1 H | 13,6 | ||||||
| 2 He | 24,6 | ||||||
| 3 Li | 54,7 | ||||||
| 4 Be | 111,5 | ||||||
| 5 B | 188 | ||||||
| 6 C | 284,2 | ||||||
| 7 N | 409,9 | 37,3 | |||||
| 8 O | 543,1 | 41,6 | |||||
| 9 F | 696,7 | ||||||
| 10 Ne | 870,2 | 48,5 | 21,7 | 21,6 | |||
| 11 Na | 1070,8 | 63,5 | 30,65 | 30,81 | |||
| 12 Mg | 1303 | 88,7 | 49,78 | 49,5 | |||
| 13 Al | 1559,6 | 117,8 | 72,95 | 72,55 | |||
| 14 Si | 1839 | 149,7 | 99,82 | 99,42 | |||
| 15 P | 2145,5 | 189 | 136 | 135 | |||
| 16 S | 2472 | 230,9 | 163,6 | 162,5 | |||
| 17 Cl | 2822,4 | 270 | 202 | 200 | |||
| 18 Ar | 3205,9 | 326,3 | 250,6 | 248,4 | 29,3 | 15,9 | 15,7 |
| 19 K | 3608,4 | 378,6 | 297,3 | 294,6 | 34,8 | 18,3 | 18,3 |
| 20 Ca | 4038,5 | 438,4 | 349,7 | 346,2 | 44,3 | 25,4 | 25,4 |
| 21 Sc | 4492 | 498 | 403,6 | 398,7 | 51,1 | 28,3 | 28,3 |
| 22 Ti | 4966 | 560,9 | 460,2 | 453,8 | 58,7 | 32,6 | 32,6 |
| 23 V | 5465 | 626,7 | 519,8 | 512,1 | 66,3 | 37,2 | 37,2 |
| 24 Cr | 5989 | 696 | 583,8 | 574,1 | 74,1 | 42,2 | 42,2 |
| 25 Mn | 6539 | 769,1 | 649,9 | 638,7 | 82,3 | 47,2 | 47,2 |
| 26 Fe | 7112 | 844,6 | 719,9 | 706,8 | 91,3 | 52,7 | 52,7 |
| 27 Co | 7709 | 925,1 | 793,2 | 778,1 | 101 | 58,9 | 59,9 |
| 28 Ni | 8333 | 1008,6 | 870 | 852,7 | 110,8 | 68 | 66,2 |
| 29 Cu | 8979 | 1096,7 | 952,3 | 932,7 | 122,5 | 77,3 | 75,1 |
Zink (30) to iodine (53)
| Orbital | 1s | 2s | 2p1/2 | 2p3/2 | 3s | 3p1/2 | 3p3/2 | 3d3/2 | 3d5/2 | 4s | 4p1/2 | 4p3/2 | 4d3/2 | 4d5/2 |
| 30 Zn | 9659 | 1196,2 | 1044,9 | 1021,8 | 139,8 | 91,4 | 88,6 | 10,2 | 10,1 | |||||
| 31 Ga | 10367 | 1299 | 1143,2 | 1116,4 | 159,5 | 103,5 | 100 | 18,7 | 18,7 | |||||
| 32 Ge | 11103 | 1414,6 | 1248,1 | 1217 | 180,1 | 124,9 | 120,8 | 29,8 | 29,2 | |||||
| 33 As | 11867 | 1527 | 1359,1 | 1323,6 | 204,7 | 146,2 | 141,2 | 41,7 | 41,7 | |||||
| 34 Se | 12658 | 1652 | 1474,3 | 1433,9 | 229,6 | 166,5 | 160,7 | 55,5 | 54,6 | |||||
| 35 Br | 13474 | 1782 | 1596 | 1550 | 257 | 189 | 182 | 70 | 69 | |||||
| 36 Kr | 14326 | 1921 | 1730,9 | 1678,4 | 292,8 | 222,2 | 214,4 | 95 | 93,8 | 27,5 | 14,1 | 14,1 | ||
| 37 Rb | 15200 | 2065 | 1864 | 1804 | 326,7 | 248,7 | 239,1 | 113 | 112 | 30,5 | 16,3 | 15,3 | ||
| 38 Sr | 16105 | 2216 | 2007 | 1940 | 358,7 | 280,3 | 270 | 136 | 134,2 | 38,9 | 21,3 | 20,1 | ||
| 39 Y | 17038 | 2373 | 2156 | 2080 | 392 | 310,6 | 298,8 | 157,7 | 155,8 | 43,8 | 24,4 | 23,1 | ||
| 40 Zr | 17998 | 2532 | 2307 | 2223 | 430,3 | 343,5 | 329,8 | 181,1 | 178,8 | 50,6 | 28,5 | 27,1 | ||
| 41 Nb | 18986 | 2698 | 2465 | 2371 | 466,6 | 376,1 | 360,6 | 205 | 202,3 | 56,4 | 32,6 | 30,8 | ||
| 42 Mo | 20000 | 2866 | 2625 | 2520 | 506,3 | 411,6 | 394 | 231,1 | 227,9 | 63,2 | 37,6 | 35,5 | ||
| 43 Tc | 21044 | 3043 | 2793 | 2677 | 544 | 447,6 | 417,7 | 257,6 | 253,9 | 69,5 | 42,3 | 39,9 | ||
| 44 Ru | 22117 | 3224 | 2967 | 2838 | 586,1 | 483,5 | 461,4 | 284,2 | 280 | 75 | 46,3 | 43,2 | ||
| 45 Rh | 23220 | 3412 | 3146 | 3004 | 628,1 | 521,3 | 496,5 | 311,9 | 307,2 | 81,4 | 50,5 | 47,3 | ||
| 46 Pd | 24350 | 3604 | 3330 | 3173 | 671,6 | 559,9 | 532,3 | 340,5 | 335,2 | 87,1 | 55,7 | 50,9 | ||
| 47 Ag | 25514 | 3806 | 3524 | 3351 | 719 | 603,8 | 573 | 374 | 368,3 | 97 | 63,7 | 58,3 | ||
| 48 Cd | 26711 | 4018 | 3727 | 3538 | 772 | 652,6 | 618,4 | 411,9 | 405,2 | 109,8 | 63,9 | 63,9 | 11,7 | 10,7 |
| 49 In | 27940 | 4238 | 3938 | 3730 | 827,2 | 703,2 | 665,3 | 451,4 | 443,9 | 122,9 | 73,5 | 73,5 | 17,7 | 16,9 |
| 50 Sn | 29200 | 4465 | 4156 | 3929 | 884,7 | 756,5 | 714,6 | 493,2 | 484,9 | 137,1 | 83,6 | 83,6 | 24,9 | 23,9 |
| 51 Sb | 30491 | 4698 | 4380 | 4132 | 946 | 812,7 | 766,4 | 537,5 | 528,2 | 153,2 | 95,6 | 95,6 | 33,3 | 32,1 |
| 52 Te | 31814 | 4939 | 4612 | 4341 | 1006 | 870,8 | 820 | 583,4 | 573 | 169,4 | 103,3 | 103,3 | 41,9 | 40,4 |
| 53 I | 33169 | 5188 | 4852 | 4557 | 1072 | 931 | 875 | 630,8 | 619,3 | 186 | 123 | 123 | 50,6 | 48,9 |
Xenon (54) to gold (79)
| Orbital | 1s | 2s | 2p1/2 | 2p3/2 | 3s | 3p1/2 | 3p3/2 | 3d3/2 | 3d5/2 | 4s | 4p1/2 | 4p3/2 | 4d3/2 | 4d5/2 | 4f5/2 | 4f7/2 | 5s | 5p1/2 | 5p3/2 |
| 54 Xe | 34561 | 5453 | 5107 | 4786 | 1148,7 | 1002,1 | 940,6 | 689 | 676,4 | 213,2 | 146,7 | 145,5 | 69,5 | 67,5 | — | — | 23,3 | 13,4 | 12,1 |
| 55 Cs | 35985 | 5714 | 5359 | 5012 | 1211 | 1071 | 1003 | 740,5 | 726,6 | 232,3 | 172,4 | 161,3 | 79,8 | 77,5 | — | — | 22,7 | 14,2 | 12,1 |
| 56 Ba | 37441 | 5989 | 5624 | 5247 | 1293 | 1137 | 1063 | 795,7 | 780,5 | 253,5 | 192 | 178,6 | 92,6 | 89,9 | — | — | 30,3 | 17 | 14,8 |
| 57 La | 38925 | 6266 | 5891 | 5483 | 1362 | 1209 | 1128 | 853 | 836 | 274,7 | 205,8 | 196 | 105,3 | 102,5 | — | — | 34,3 | 19,3 | 16,8 |
| 58 Ce | 40443 | 6549 | 6164 | 5723 | 1436 | 1274 | 1187 | 902,4 | 883,8 | 291 | 223,2 | 206,5 | 109 | — | 0,1 | 0,1 | 37,8 | 19,8 | 17 |
| 59 Pr | 41991 | 6835 | 6440 | 5964 | 1511 | 1337 | 1242 | 948,3 | 928,8 | 304,5 | 236,3 | 217,6 | 115,1 | 115,1 | 2 | 2 | 37,4 | 22,3 | 22,3 |
| 60 Nd | 43569 | 7126 | 6722 | 6208 | 1575 | 1403 | 1297 | 1003,3 | 980,4 | 319,2 | 243,3 | 224,6 | 120,5 | 120,5 | 1,5 | 1,5 | 37,5 | 21,1 | 21,1 |
| 61 Pm | 45184 | 7428 | 7013 | 6459 | 1471 | 1357 | 1052 | 1027 | 242 | 242 | 120 | 120 | — | — | — | — | — | ||
| 62 Sm | 46834 | 7737 | 7312 | 6716 | 1723 | 1541 | 1420 | 1110,9 | 1083,4 | 347,2 | 265,6 | 247,4 | 129 | 129 | 5,2 | 5,2 | 37,4 | 21,3 | 21,3 |
| 63 Eu | 48519 | 8052 | 7617 | 6977 | 1800 | 1614 | 1481 | 1158,6 | 1127,5 | 360 | 284 | 257 | 133 | 127,7 | 0 | 0 | 32 | 22 | 22 |
| 64 Gd | 50239 | 8376 | 7930 | 7243 | 1881 | 1688 | 1544 | 1221,9 | 1189,6 | 378,6 | 286 | 271 | — | 142,6 | 8,6 | 8,6 | 36 | 28 | 21 |
| 65 Tb | 51996 | 8708 | 8252 | 7514 | 1968 | 1768 | 1611 | 1276,9 | 1241,1 | 396 | 322,4 | 284,1 | 150,5 | 150,5 | 7,7 | 2,4 | 45,6 | 28,7 | 22,6 |
| 66 Dy | 53789 | 9046 | 8581 | 7790 | 2047 | 1842 | 1676 | 1333 | 1292,6 | 414,2 | 333,5 | 293,2 | 153,6 | 153,6 | 8 | 4,3 | 49,9 | 26,3 | 26,3 |
| 67 Ho | 55618 | 9394 | 8918 | 8071 | 2128 | 1923 | 1741 | 1392 | 1351 | 432,4 | 343,5 | 308,2 | 160 | 160 | 8,6 | 5,2 | 49,3 | 30,8 | 24,1 |
| 68 Er | 57486 | 9751 | 9264 | 8358 | 2207 | 2006 | 1812 | 1453 | 1409 | 449,8 | 366,2 | 320,2 | 167,6 | 167,6 | — | 4,7 | 50,6 | 31,4 | 24,7 |
| 69 Tm | 59390 | 10116 | 9617 | 8648 | 2307 | 2090 | 1885 | 1515 | 1468 | 470,9 | 385,9 | 332,6 | 175,5 | 175,5 | — | 4,6 | 54,7 | 31,8 | 25 |
| 70 Yb | 61332 | 10486 | 9978 | 8944 | 2398 | 2173 | 1950 | 1576 | 1528 | 480,5 | 388,7 | 339,7 | 191,2 | 182,4 | 2,5 | 1,3 | 52 | 30,3 | 24,1 |
| 71 Lu | 63314 | 10870 | 10349 | 9244 | 2491 | 2264 | 2024 | 1639 | 1589 | 506,8 | 412,4 | 359,2 | 206,1 | 196,3 | 8,9 | 7,5 | 57,3 | 33,6 | 26,7 |
| 72 Hf | 65351 | 11271 | 10739 | 9561 | 2601 | 2365 | 2108 | 1716 | 1662 | 538 | 438,2 | 380,7 | 220 | 211,5 | 15,9 | 14,2 | 64,2 | 38 | 29,9 |
| 73 Ta | 67416 | 11682 | 11136 | 9881 | 2708 | 2469 | 2194 | 1793 | 1735 | 563,4 | 463,4 | 400,9 | 237,9 | 226,4 | 23,5 | 21,6 | 69,7 | 42,2 | 32,7 |
| 74 W | 69525 | 12100 | 11544 | 10207 | 2820 | 2575 | 2281 | 1872 | 1809 | 594,1 | 490,4 | 423,6 | 255,9 | 243,5 | 33,6 | 31,4 | 75,6 | 45,3 | 36,8 |
| 75 Re | 71676 | 12527 | 11959 | 10535 | 2932 | 2682 | 2367 | 1949 | 1883 | 625,4 | 518,7 | 446,8 | 273,9 | 260,5 | 42,9 | 40,5 | 83 | 45,6 | 34,6 |
| 76 Os | 73871 | 12968 | 12385 | 10871 | 3049 | 2792 | 2457 | 2031 | 1960 | 658,2 | 549,1 | 470,7 | 293,1 | 278,5 | 53,4 | 50,7 | 84 | 58 | 44,5 |
| 77 Ir | 76111 | 13419 | 12824 | 11215 | 3174 | 2909 | 2551 | 2116 | 2040 | 691,1 | 577,8 | 495,8 | 311,9 | 296,3 | 63,8 | 60,8 | 95,2 | 63 | 48 |
| 78 Pt | 78395 | 13880 | 13273 | 11564 | 3296 | 3027 | 2645 | 2202 | 2122 | 725,4 | 609,1 | 519,4 | 331,6 | 314,6 | 74,5 | 71,2 | 101,7 | 65,3 | 51,7 |
| 79 Au | 80725 | 14353 | 13734 | 11919 | 3425 | 3148 | 2743 | 2291 | 2206 | 762,1 | 642,7 | 546,3 | 353,2 | 335,1 | 87,6 | 84 | 107,2 | 74,2 | 57,2 |
Mercury (80) to uranium (92)
| Orbital | 1s | 2s | 2p1/2 | 2p3/2 | 3s | 3p1/2 | 3p3/2 | 3d3/2 | 3d5/2 | 4s | 4p1/2 | 4p3/2 | 4d3/2 | 4d5/2 | 4f5/2 | 4f7/2 | 5s | 5p1/2 | 5p3/2 | 5d3/2 | 5d5/2 | 6s | 6p1/2 | 6p3/2 |
| 80 Hg | 83102 | 14839 | 14209 | 12284 | 3562 | 3279 | 2847 | 2385 | 2295 | 802,2 | 680,2 | 576,6 | 378,2 | 358,8 | 104 | 99,9 | 127 | 83,1 | 64,5 | 9,6 | 7,8 | |||
| 81 Tl | 85530 | 15347 | 14698 | 12658 | 3704 | 3416 | 2957 | 2485 | 2389 | 846,2 | 720,5 | 609,5 | 405,7 | 385 | 122,2 | 117,8 | 136 | 94,6 | 73,5 | 14,7 | 12,5 | |||
| 82 Pb | 88005 | 15861 | 15200 | 13035 | 3851 | 3554 | 3066 | 2586 | 2484 | 891,8 | 761,9 | 643,5 | 434,3 | 412,2 | 141,7 | 136,9 | 147 | 106,4 | 83,3 | 20,7 | 18,1 | |||
| 83 Bi | 90524 | 16388 | 15711 | 13419 | 3999 | 3696 | 3177 | 2688 | 2580 | 939 | 805,2 | 678,8 | 464 | 440,1 | 162,3 | 157 | 159,3 | 119 | 92,6 | 26,9 | 23,8 | |||
| 84 Po | 93105 | 16939 | 16244 | 13814 | 4149 | 3854 | 3302 | 2798 | 2683 | 995 | 851 | 705 | 500 | 473 | 184 | 184 | 177 | 132 | 104 | 31 | 31 | |||
| 85 At | 95730 | 17493 | 16785 | 14214 | 4317 | 4008 | 3426 | 2909 | 2787 | 1042 | 886 | 740 | 533 | 507 | 210 | 210 | 195 | 148 | 115 | 40 | 40 | |||
| 86 Rn | 98404 | 18049 | 17337 | 14619 | 4482 | 4159 | 3538 | 3022 | 2892 | 1097 | 929 | 768 | 567 | 541 | 238 | 238 | 214 | 164 | 127 | 48 | 48 | 26 | ||
| 87 Fr | 101137 | 18639 | 17907 | 15031 | 4652 | 4327 | 3663 | 3136 | 3000 | 1153 | 980 | 810 | 603 | 577 | 268 | 268 | 234 | 182 | 140 | 58 | 58 | 34 | 15 | 15 |
| 88 Ra | 103922 | 19237 | 18484 | 15444 | 4822 | 4490 | 3792 | 3248 | 3105 | 1208 | 1058 | 879 | 636 | 603 | 299 | 299 | 254 | 200 | 153 | 68 | 68 | 44 | 19 | 19 |
| 89 Ac | 106755 | 19840 | 19083 | 15871 | 5002 | 4656 | 3909 | 3370 | 3219 | 1269 | 1080 | 890 | 675 | 639 | 319 | 319 | 272 | 215 | 167 | 80 | 80 | — | — | - |
| 90 Th | 109651 | 20472 | 19693 | 16300 | 5182 | 4830 | 4046 | 3491 | 3332 | 1330 | 1168 | 966,4 | 712,1 | 675,2 | 342,4 | 333,1 | 290 | 229 | 182 | 92,5 | 85,4 | 41,4 | 24,5 | 16,6 |
| 91 Pa | 112601 | 21105 | 20314 | 16733 | 5367 | 5001 | 4174 | 3611 | 3442 | 1387 | 1224 | 1007 | 743 | 708 | 371 | 360 | 310 | 232 | 232 | 94 | 94 | — | — | - |
| 92 U | 115606 | 21757 | 20948 | 17166 | 5548 | 5182 | 4303 | 3728 | 3552 | 1439 | 1271 | 1043 | 778,3 | 736,2 | 388,2 | 377,4 | 321 | 257 | 192 | 102,8 | 94,2 | 43,9 | 26,8 | 16,8 |