Specific Process Knowledge/Characterization/XPS/XPS elemental composition: Difference between revisions
| (58 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Feedback to this page''': '''[mailto:labadviser@ | '''Feedback to this page''': '''[mailto:labadviser@nanolab.dtu.dk?Subject=Feed%20back%20from%20page%20http://labadviser.nanolab.dtu.dk/index.php?title=Specific_Process_Knowledge/Characterization/XPS/XPS_elemental_composition click here]''' | ||
<!-- Page reviewed 9/8-2022 jmli --> | |||
=Elemental composition analysis= | =Elemental composition analysis= | ||
{{Author-jmli1}} | |||
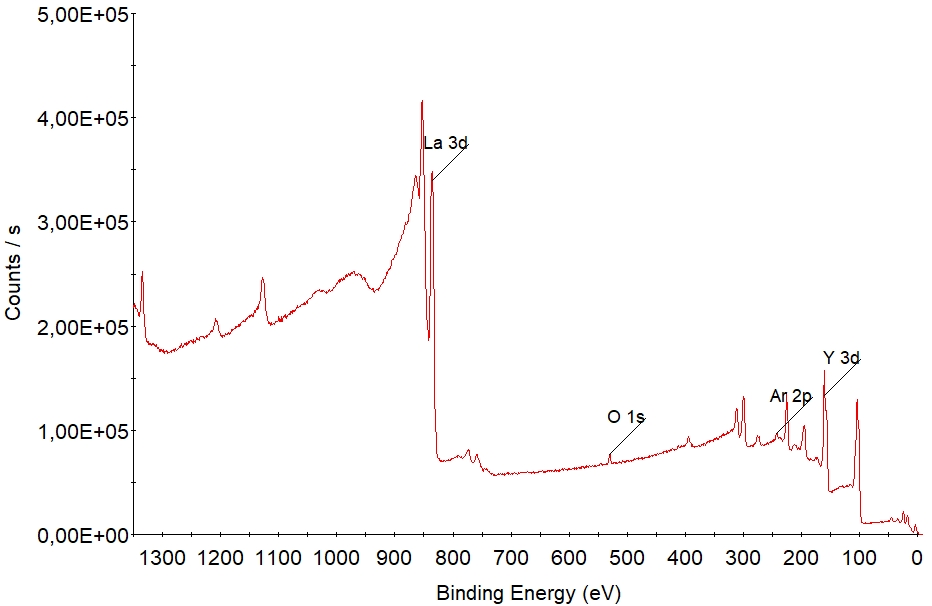
[[File: | [[File:XPS survey.jpg|XPS spectrum of a sample consisting of the elements silicon, oxygen and carbon. {{image1}}]] | ||
With a unique set of electronic states, each element has its own "finger-print" in the XPS spectrum. | With a unique set of electronic states, each element has its own "finger-print" in the XPS spectrum. The electronic states in the atoms are occupied by electrons with a specific binding energy. In the spectrum above, the dominant peaks of carbon, oxygen and silicon are labelled. More peaks are seen in this socalled survey spectrum but they are all associated to these three elements. All elements have peaks within the wide energy range (usually 0 - 1350 eV binding energy) of the survey spectrum - hence any element that is present in the sample within its detection limit (roughly 1 % depending on element and acquisition setup) will show up in the survey spectrum as peaks of intensities that are determined by the respective concentrations. | ||
As such, survey spectra are used to determined what elements are present in the sample. | |||
For each element identified in the survey spectrum, one typically runs a spectrum at the dominant peak. These spectra have a much narrower energy ranges and therefore much smaller energy steps - this enables us to achieve much better energy resolution and hence to see the finer details of each element. These spectra, called high resolution spectra, contain the chemical information that is the strong selling point of the XPS technique. | |||
== Table with approximate binding energies == | |||
The table below has been adapted from the web archive at https://xdb.lbl.gov/Section1/Sec_1-1.html. | |||
=Hydrogen (1) to copper (29)= | |||
{| border="2" cellspacing="0" cellpadding="1" {{table}} | {| border="2" cellspacing="0" cellpadding="1" {{table}} | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''Orbital''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''1s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''2p<sub>3/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3s''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>1/2</sub>''' | ||
| align="center" style="background:#f0f0f0;"|''' | | align="center" style="background:#f0f0f0;"|'''3p<sub>3/2</sub>''' | ||
| | |- | ||
| | | ''' 1 H'''||13,6|||||||||||| | ||
| | |- | ||
| | | '''2 He'''||24,6|||||||||||| | ||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
|- | |- | ||
| | | '''3 Li'''||54,7|||||||||||| | ||
|- | |- | ||
| | | '''4 Be'''||111,5|||||||||||| | ||
|- | |- | ||
| | | ''' 5 B'''||188|||||||||||| | ||
|- | |- | ||
| | | '''6 C'''||284,2|||||||||||| | ||
|- | |- | ||
| | | '''7 N'''||409,9||37,3|||||||||| | ||
|- | |- | ||
| | | '''8 O'''||543,1||41,6|||||||||| | ||
|- | |- | ||
| | | '''9 F'''||696,7|||||||||||| | ||
|- | |- | ||
| | | '''10 Ne'''||870,2||48,5||21,7||21,6|||||| | ||
|- | |- | ||
| | | '''11 Na'''||1070,8||63,5||30,65||30,81|||||| | ||
|- | |- | ||
| | | '''12 Mg'''||1303||88,7||49,78||49,5|||||| | ||
|- | |- | ||
| | | '''13 Al'''||1559,6||117,8||72,95||72,55|||||| | ||
|- | |- | ||
| | | '''14 Si'''||1839||149,7||99,82||99,42|||||| | ||
|- | |- | ||
| | | '''15 P'''||2145,5||189||136||135|||||| | ||
|- | |- | ||
| | | '''16 S'''||2472||230,9||163,6||162,5|||||| | ||
|- | |- | ||
| | | '''17 Cl'''||2822,4||270||202||200|||||| | ||
|- | |- | ||
| | | '''18 Ar'''||3205,9||326,3||250,6||248,4||29,3||15,9||15,7 | ||
|- | |- | ||
| | |''' 19 K'''||3608,4||378,6||297,3||294,6||34,8||18,3||18,3 | ||
|- | |- | ||
| | | '''20 Ca'''||4038,5||438,4||349,7||346,2||44,3||25,4||25,4 | ||
|- | |- | ||
| | |''' 21 Sc'''||4492||498||403,6||398,7||51,1||28,3||28,3 | ||
|- | |- | ||
| | | '''22 Ti'''||4966||560,9||460,2||453,8||58,7||32,6||32,6 | ||
|- | |- | ||
| | | '''23 V'''||5465||626,7||519,8||512,1||66,3||37,2||37,2 | ||
|- | |- | ||
| | | '''24 Cr'''||5989||696||583,8||574,1||74,1||42,2||42,2 | ||
|- | |- | ||
| | | '''25 Mn'''||6539||769,1||649,9||638,7||82,3||47,2||47,2 | ||
|- | |- | ||
| | | '''26 Fe'''||7112||844,6||719,9||706,8||91,3||52,7||52,7 | ||
|- | |- | ||
| | | '''27 Co'''||7709||925,1||793,2||778,1||101||58,9||59,9 | ||
|- | |- | ||
| | | '''28 Ni'''||8333||1008,6||870||852,7||110,8||68||66,2 | ||
|- | |- | ||
| | | '''29 Cu'''||8979||1096,7||952,3||932,7||122,5||77,3||75,1 | ||
|- | |- | ||
| | |} | ||
= Zink (30) to iodine (53) = | |||
{| border="2" cellspacing="0" cellpadding="1" {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Orbital''' | |||
| align="center" style="background:#f0f0f0;"|'''1s''' | |||
| align="center" style="background:#f0f0f0;"|'''2s''' | |||
| align="center" style="background:#f0f0f0;"|'''2p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''2p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3s''' | |||
| align="center" style="background:#f0f0f0;"|'''3p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3d<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3d<sub>5/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4s''' | |||
| align="center" style="background:#f0f0f0;"|'''4p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4d<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4d<sub>5/2</sub>''' | |||
|- | |- | ||
| | | '''30 Zn'''||9659||1196,2||1044,9||1021,8||139,8||91,4||88,6||10,2||10,1|||||||||| | ||
|- | |- | ||
| | | '''31 Ga'''||10367||1299||1143,2||1116,4||159,5||103,5||100||18,7||18,7|||||||||| | ||
|- | |- | ||
| | | '''32 Ge'''||11103||1414,6||1248,1||1217||180,1||124,9||120,8||29,8||29,2|||||||||| | ||
|- | |- | ||
| | | '''33 As'''||11867||1527||1359,1||1323,6||204,7||146,2||141,2||41,7||41,7|||||||||| | ||
|- | |- | ||
| | | '''34 Se'''||12658||1652||1474,3||1433,9||229,6||166,5||160,7||55,5||54,6|||||||||| | ||
|- | |- | ||
| | | '''35 Br'''||13474||1782||1596||1550||257||189||182||70||69|||||||||| | ||
|- | |- | ||
| | | '''36 Kr'''||14326||1921||1730,9||1678,4||292,8||222,2||214,4||95||93,8||27,5||14,1||14,1|||| | ||
|- | |- | ||
| | | '''37 Rb'''||15200||2065||1864||1804||326,7||248,7||239,1||113||112||30,5||16,3||15,3|||| | ||
|- | |- | ||
| '''38 Sr'''||16105||2216||2007||1940||358,7||280,3||270||136||134,2||38,9||21,3||20,1|||| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
|- | |- | ||
| | | '''39 Y'''||17038||2373||2156||2080||392||310,6||298,8||157,7||155,8||43,8||24,4||23,1|||| | ||
|- | |- | ||
| | | '''40 Zr'''||17998||2532||2307||2223||430,3||343,5||329,8||181,1||178,8||50,6||28,5||27,1|||| | ||
|- | |- | ||
| | | '''41 Nb'''||18986||2698||2465||2371||466,6||376,1||360,6||205||202,3||56,4||32,6||30,8|||| | ||
|- | |- | ||
| | |''' 42 Mo'''||20000||2866||2625||2520||506,3||411,6||394||231,1||227,9||63,2||37,6||35,5|||| | ||
|- | |- | ||
| | |''' 43 Tc'''||21044||3043||2793||2677||544||447,6||417,7||257,6||253,9||69,5||42,3||39,9|||| | ||
|- | |- | ||
| | |''' 44 Ru'''||22117||3224||2967||2838||586,1||483,5||461,4||284,2||280||75||46,3||43,2|||| | ||
|- | |- | ||
| | | '''45 Rh'''||23220||3412||3146||3004||628,1||521,3||496,5||311,9||307,2||81,4||50,5||47,3|||| | ||
|- | |- | ||
| | | '''46 Pd'''||24350||3604||3330||3173||671,6||559,9||532,3||340,5||335,2||87,1||55,7||50,9|||| | ||
|- | |- | ||
| | | '''47 Ag'''||25514||3806||3524||3351||719||603,8||573||374||368,3||97||63,7||58,3|||| | ||
|- | |- | ||
| | | '''48 Cd'''||26711||4018||3727||3538||772||652,6||618,4||411,9||405,2||109,8||63,9||63,9||11,7||10,7 | ||
|- | |- | ||
| | | '''49 In'''||27940||4238||3938||3730||827,2||703,2||665,3||451,4||443,9||122,9||73,5||73,5||17,7||16,9 | ||
|- | |- | ||
| | | '''50 Sn'''||29200||4465||4156||3929||884,7||756,5||714,6||493,2||484,9||137,1||83,6||83,6||24,9||23,9 | ||
|- | |- | ||
| | | '''51 Sb'''||30491||4698||4380||4132||946||812,7||766,4||537,5||528,2||153,2||95,6||95,6||33,3||32,1 | ||
|- | |- | ||
| | | '''52 Te'''||31814||4939||4612||4341||1006||870,8||820||583,4||573||169,4||103,3||103,3||41,9||40,4 | ||
|- | |- | ||
| | | '''53 I'''||33169||5188||4852||4557||1072||931||875||630,8||619,3||186||123||123||50,6||48,9 | ||
|- | |- | ||
| | |} | ||
=Xenon (54) to gold (79) = | |||
{| border="2" cellspacing="0" cellpadding="1" {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Orbital''' | |||
| align="center" style="background:#f0f0f0;"|'''1s''' | |||
| align="center" style="background:#f0f0f0;"|'''2s''' | |||
| align="center" style="background:#f0f0f0;"|'''2p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''2p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3s''' | |||
| align="center" style="background:#f0f0f0;"|'''3p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3d<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3d<sub>5/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4s''' | |||
| align="center" style="background:#f0f0f0;"|'''4p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4d<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4d<sub>5/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4f<sub>5/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4f<sub>7/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''5s''' | |||
| align="center" style="background:#f0f0f0;"|'''5p<sub>1/2</sub> ''' | |||
| align="center" style="background:#f0f0f0;"|'''5p<sub>3/2</sub>''' | |||
|- | |- | ||
| | | '''54 Xe'''||34561||5453||5107||4786||1148,7||1002,1||940,6||689||676,4||213,2||146,7||145,5||69,5||67,5||—||—||23,3||13,4||12,1 | ||
|- | |- | ||
| | | '''55 Cs'''||35985||5714||5359||5012||1211||1071||1003||740,5||726,6||232,3||172,4||161,3||79,8||77,5||—||—||22,7||14,2||12,1 | ||
|- | |- | ||
| | | '''56 Ba'''||37441||5989||5624||5247||1293||1137||1063||795,7||780,5||253,5||192||178,6||92,6||89,9||—||—||30,3||17||14,8 | ||
|- | |- | ||
| | | '''57 La'''||38925||6266||5891||5483||1362||1209||1128||853||836||274,7||205,8||196||105,3||102,5||—||—||34,3||19,3||16,8 | ||
|- | |- | ||
| | | '''58 Ce'''||40443||6549||6164||5723||1436||1274||1187||902,4||883,8||291||223,2||206,5||109||—||0,1||0,1||37,8||19,8||17 | ||
|- | |- | ||
| | | '''59 Pr'''||41991||6835||6440||5964||1511||1337||1242||948,3||928,8||304,5||236,3||217,6||115,1||115,1||2||2||37,4||22,3||22,3 | ||
|- | |- | ||
| | | '''60 Nd'''||43569||7126||6722||6208||1575||1403||1297||1003,3||980,4||319,2||243,3||224,6||120,5||120,5||1,5||1,5||37,5||21,1||21,1 | ||
|- | |- | ||
| | | '''61 Pm'''||45184||7428||7013||6459||||1471||1357||1052||1027||||242||242||120||120||—||—||—||—||— | ||
|- | |- | ||
| | | '''62 Sm''' ||46834||7737||7312||6716||1723||1541||1420||1110,9||1083,4||347,2||265,6||247,4||129||129||5,2||5,2||37,4||21,3||21,3 | ||
|- | |- | ||
| | | '''63 Eu'''||48519||8052||7617||6977||1800||1614||1481||1158,6||1127,5||360||284||257||133||127,7||0||0||32||22||22 | ||
|- | |- | ||
| | | '''64 Gd'''||50239||8376||7930||7243||1881||1688||1544||1221,9||1189,6||378,6||286||271||—||142,6||8,6||8,6||36||28||21 | ||
|- | |- | ||
| | | '''65 Tb'''||51996||8708||8252||7514||1968||1768||1611||1276,9||1241,1||396||322,4||284,1||150,5||150,5||7,7||2,4||45,6||28,7||22,6 | ||
|- | |- | ||
| | | '''66 Dy'''||53789||9046||8581||7790||2047||1842||1676||1333||1292,6||414,2||333,5||293,2||153,6||153,6||8||4,3||49,9||26,3||26,3 | ||
|- | |- | ||
| | | '''67 Ho'''||55618||9394||8918||8071||2128||1923||1741||1392||1351||432,4||343,5||308,2||160||160||8,6||5,2||49,3||30,8||24,1 | ||
|- | |- | ||
| | | '''68 Er'''||57486||9751||9264||8358||2207||2006||1812||1453||1409||449,8||366,2||320,2||167,6||167,6||—||4,7||50,6||31,4||24,7 | ||
|- | |- | ||
| | | '''69 Tm'''||59390||10116||9617||8648||2307||2090||1885||1515||1468||470,9||385,9||332,6||175,5||175,5||—||4,6||54,7||31,8||25 | ||
|- | |- | ||
| | | '''70 Yb'''||61332||10486||9978||8944||2398||2173||1950||1576||1528||480,5||388,7||339,7||191,2||182,4||2,5||1,3||52||30,3||24,1 | ||
|- | |- | ||
| | | '''71 Lu'''||63314||10870||10349||9244||2491||2264||2024||1639||1589||506,8||412,4||359,2||206,1||196,3||8,9||7,5||57,3||33,6||26,7 | ||
|- | |- | ||
| | | '''72 Hf'''||65351||11271||10739||9561||2601||2365||2108||1716||1662||538||438,2||380,7||220||211,5||15,9||14,2||64,2||38||29,9 | ||
|- | |- | ||
| | | '''73 Ta'''||67416||11682||11136||9881||2708||2469||2194||1793||1735||563,4||463,4||400,9||237,9||226,4||23,5||21,6||69,7||42,2||32,7 | ||
|- | |- | ||
| | | '''74 W'''||69525||12100||11544||10207||2820||2575||2281||1872||1809||594,1||490,4||423,6||255,9||243,5||33,6||31,4||75,6||45,3||36,8 | ||
|- | |- | ||
| | | '''75 Re'''||71676||12527||11959||10535||2932||2682||2367||1949||1883||625,4||518,7||446,8||273,9||260,5||42,9||40,5||83||45,6||34,6 | ||
|- | |- | ||
| | | '''76 Os'''||73871||12968||12385||10871||3049||2792||2457||2031||1960||658,2||549,1||470,7||293,1||278,5||53,4||50,7||84||58||44,5 | ||
|- | |- | ||
| | | '''77 Ir'''||76111||13419||12824||11215||3174||2909||2551||2116||2040||691,1||577,8||495,8||311,9||296,3||63,8||60,8||95,2||63||48 | ||
|- | |- | ||
| | | '''78 Pt'''||78395||13880||13273||11564||3296||3027||2645||2202||2122||725,4||609,1||519,4||331,6||314,6||74,5||71,2||101,7||65,3||51,7 | ||
|- | |- | ||
| | | '''79 Au'''||80725||14353||13734||11919||3425||3148||2743||2291||2206||762,1||642,7||546,3||353,2||335,1||87,6||84||107,2||74,2||57,2 | ||
|- | |- | ||
| | |} | ||
= Mercury (80) to uranium (92)= | |||
{| border="2" cellspacing="0" cellpadding="1" {{table}} | |||
| align="center" style="background:#f0f0f0;"|'''Orbital''' | |||
| align="center" style="background:#f0f0f0;"|'''1s''' | |||
| align="center" style="background:#f0f0f0;"|'''2s''' | |||
| align="center" style="background:#f0f0f0;"|'''2p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''2p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3s''' | |||
| align="center" style="background:#f0f0f0;"|'''3p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3d<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''3d<sub>5/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4s''' | |||
| align="center" style="background:#f0f0f0;"|'''4p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4d<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4d<sub>5/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4f<sub>5/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''4f<sub>7/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''5s''' | |||
| align="center" style="background:#f0f0f0;"|'''5p<sub>1/2</sub> ''' | |||
| align="center" style="background:#f0f0f0;"|'''5p<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''5d<sub>3/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''5d<sub>5/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''6s''' | |||
| align="center" style="background:#f0f0f0;"|'''6p<sub>1/2</sub>''' | |||
| align="center" style="background:#f0f0f0;"|'''6p<sub>3/2</sub>''' | |||
|- | |- | ||
| 80 | | '''80 Hg'''||83102||14839||14209||12284||3562||3279||2847||2385||2295||802,2||680,2||576,6||378,2||358,8||104||99,9||127||83,1||64,5||9,6||7,8|||||| | ||
|- | |- | ||
| 81 Tl||85530||15347||14698||12658||3704||3416||2957||2485||2389||846,2||720,5||609,5||405,7||385||122,2||117,8||136||94,6||73,5||14,7||12,5|||| | | '''81 Tl'''||85530||15347||14698||12658||3704||3416||2957||2485||2389||846,2||720,5||609,5||405,7||385||122,2||117,8||136||94,6||73,5||14,7||12,5|||||| | ||
|- | |- | ||
| 82 Pb||88005||15861||15200||13035||3851||3554||3066||2586||2484||891,8||761,9||643,5||434,3||412,2||141,7||136,9||147||106,4||83,3||20,7||18,1|||| | | '''82 Pb'''||88005||15861||15200||13035||3851||3554||3066||2586||2484||891,8||761,9||643,5||434,3||412,2||141,7||136,9||147||106,4||83,3||20,7||18,1|||||| | ||
|- | |- | ||
| 83 Bi||90524||16388||15711||13419||3999||3696||3177||2688||2580||939||805,2||678,8||464||440,1||162,3||157||159,3||119||92,6||26,9||23,8|||| | | '''83 Bi'''||90524||16388||15711||13419||3999||3696||3177||2688||2580||939||805,2||678,8||464||440,1||162,3||157||159,3||119||92,6||26,9||23,8|||||| | ||
|- | |- | ||
| 84 Po||93105||16939||16244||13814||4149||3854||3302||2798||2683||995||851||705||500||473||184||184||177||132||104||31||31|||| | | '''84 Po'''||93105||16939||16244||13814||4149||3854||3302||2798||2683||995||851||705||500||473||184||184||177||132||104||31||31|||||| | ||
|- | |- | ||
| 85 At||95730||17493||16785||14214||4317||4008||3426||2909||2787||1042||886||740||533||507||210||210||195||148||115||40||40|||| | | '''85 At'''||95730||17493||16785||14214||4317||4008||3426||2909||2787||1042||886||740||533||507||210||210||195||148||115||40||40|||||| | ||
|- | |- | ||
| 86 Rn||98404||18049||17337||14619||4482||4159||3538||3022||2892||1097||929||768||567||541||238||238||214||164||127||48||48||26|| | | '''86 Rn'''||98404||18049||17337||14619||4482||4159||3538||3022||2892||1097||929||768||567||541||238||238||214||164||127||48||48||26|||| | ||
|- | |- | ||
| 87 Fr||101137||18639||17907||15031||4652||4327||3663||3136||3000||1153||980||810||603||577||268||268||234||182||140||58||58||34||15 | | '''87 Fr'''||101137||18639||17907||15031||4652||4327||3663||3136||3000||1153||980||810||603||577||268||268||234||182||140||58||58||34||15||15 | ||
|- | |- | ||
| 88 Ra||103922||19237||18484||15444||4822||4490||3792||3248||3105||1208||1058||879||636||603||299||299||254||200||153||68||68||44||19 | | '''88 Ra'''||103922||19237||18484||15444||4822||4490||3792||3248||3105||1208||1058||879||636||603||299||299||254||200||153||68||68||44||19|| 19 | ||
|- | |- | ||
| 89 Ac||106755||19840||19083||15871||5002||4656||3909||3370||3219||1269||1080||890||675||639||319||319||272||215||167||80||80||—||— | | '''89 Ac'''||106755||19840||19083||15871||5002||4656||3909||3370||3219||1269||1080||890||675||639||319||319||272||215||167||80||80||—||—||- | ||
|- | |- | ||
| 90 Th||109651||20472||19693||16300||5182||4830||4046||3491||3332||1330||1168||966,4||712,1||675,2||342,4||333,1||290||229||182||92,5||85,4||41,4||24,5 | | '''90 Th'''||109651||20472||19693||16300||5182||4830||4046||3491||3332||1330||1168||966,4||712,1||675,2||342,4||333,1||290||229||182||92,5||85,4||41,4||24,5||16,6 | ||
|- | |- | ||
| 91 Pa||112601||21105||20314||16733||5367||5001||4174||3611||3442||1387||1224||1007||743||708||371||360||310||232||232||94||94||—||— | | '''91 Pa'''||112601||21105||20314||16733||5367||5001||4174||3611||3442||1387||1224||1007||743||708||371||360||310||232||232||94||94||—||—||- | ||
|- | |- | ||
| 92 U||115606||21757||20948||17166||5548||5182||4303||3728||3552||1439||1271||1043||778,3||736,2||388,2||377,4||321||257||192||102,8||94,2||43,9||26,8 | | '''92 U'''||115606||21757||20948||17166||5548||5182||4303||3728||3552||1439||1271||1043||778,3||736,2||388,2||377,4||321||257||192||102,8||94,2||43,9||26,8||16,8 | ||
|- | |- | ||
|} | |} | ||
Revision as of 14:16, 8 May 2023
Feedback to this page: click here
Elemental composition analysis
Unless otherwise stated, all content on this page was created by Jonas Michael-Lindhard, DTU Nanolab
With a unique set of electronic states, each element has its own "finger-print" in the XPS spectrum. The electronic states in the atoms are occupied by electrons with a specific binding energy. In the spectrum above, the dominant peaks of carbon, oxygen and silicon are labelled. More peaks are seen in this socalled survey spectrum but they are all associated to these three elements. All elements have peaks within the wide energy range (usually 0 - 1350 eV binding energy) of the survey spectrum - hence any element that is present in the sample within its detection limit (roughly 1 % depending on element and acquisition setup) will show up in the survey spectrum as peaks of intensities that are determined by the respective concentrations. As such, survey spectra are used to determined what elements are present in the sample.
For each element identified in the survey spectrum, one typically runs a spectrum at the dominant peak. These spectra have a much narrower energy ranges and therefore much smaller energy steps - this enables us to achieve much better energy resolution and hence to see the finer details of each element. These spectra, called high resolution spectra, contain the chemical information that is the strong selling point of the XPS technique.
Table with approximate binding energies
The table below has been adapted from the web archive at https://xdb.lbl.gov/Section1/Sec_1-1.html.
Hydrogen (1) to copper (29)
| Orbital | 1s | 2s | 2p1/2 | 2p3/2 | 3s | 3p1/2 | 3p3/2 |
| 1 H | 13,6 | ||||||
| 2 He | 24,6 | ||||||
| 3 Li | 54,7 | ||||||
| 4 Be | 111,5 | ||||||
| 5 B | 188 | ||||||
| 6 C | 284,2 | ||||||
| 7 N | 409,9 | 37,3 | |||||
| 8 O | 543,1 | 41,6 | |||||
| 9 F | 696,7 | ||||||
| 10 Ne | 870,2 | 48,5 | 21,7 | 21,6 | |||
| 11 Na | 1070,8 | 63,5 | 30,65 | 30,81 | |||
| 12 Mg | 1303 | 88,7 | 49,78 | 49,5 | |||
| 13 Al | 1559,6 | 117,8 | 72,95 | 72,55 | |||
| 14 Si | 1839 | 149,7 | 99,82 | 99,42 | |||
| 15 P | 2145,5 | 189 | 136 | 135 | |||
| 16 S | 2472 | 230,9 | 163,6 | 162,5 | |||
| 17 Cl | 2822,4 | 270 | 202 | 200 | |||
| 18 Ar | 3205,9 | 326,3 | 250,6 | 248,4 | 29,3 | 15,9 | 15,7 |
| 19 K | 3608,4 | 378,6 | 297,3 | 294,6 | 34,8 | 18,3 | 18,3 |
| 20 Ca | 4038,5 | 438,4 | 349,7 | 346,2 | 44,3 | 25,4 | 25,4 |
| 21 Sc | 4492 | 498 | 403,6 | 398,7 | 51,1 | 28,3 | 28,3 |
| 22 Ti | 4966 | 560,9 | 460,2 | 453,8 | 58,7 | 32,6 | 32,6 |
| 23 V | 5465 | 626,7 | 519,8 | 512,1 | 66,3 | 37,2 | 37,2 |
| 24 Cr | 5989 | 696 | 583,8 | 574,1 | 74,1 | 42,2 | 42,2 |
| 25 Mn | 6539 | 769,1 | 649,9 | 638,7 | 82,3 | 47,2 | 47,2 |
| 26 Fe | 7112 | 844,6 | 719,9 | 706,8 | 91,3 | 52,7 | 52,7 |
| 27 Co | 7709 | 925,1 | 793,2 | 778,1 | 101 | 58,9 | 59,9 |
| 28 Ni | 8333 | 1008,6 | 870 | 852,7 | 110,8 | 68 | 66,2 |
| 29 Cu | 8979 | 1096,7 | 952,3 | 932,7 | 122,5 | 77,3 | 75,1 |
Zink (30) to iodine (53)
| Orbital | 1s | 2s | 2p1/2 | 2p3/2 | 3s | 3p1/2 | 3p3/2 | 3d3/2 | 3d5/2 | 4s | 4p1/2 | 4p3/2 | 4d3/2 | 4d5/2 |
| 30 Zn | 9659 | 1196,2 | 1044,9 | 1021,8 | 139,8 | 91,4 | 88,6 | 10,2 | 10,1 | |||||
| 31 Ga | 10367 | 1299 | 1143,2 | 1116,4 | 159,5 | 103,5 | 100 | 18,7 | 18,7 | |||||
| 32 Ge | 11103 | 1414,6 | 1248,1 | 1217 | 180,1 | 124,9 | 120,8 | 29,8 | 29,2 | |||||
| 33 As | 11867 | 1527 | 1359,1 | 1323,6 | 204,7 | 146,2 | 141,2 | 41,7 | 41,7 | |||||
| 34 Se | 12658 | 1652 | 1474,3 | 1433,9 | 229,6 | 166,5 | 160,7 | 55,5 | 54,6 | |||||
| 35 Br | 13474 | 1782 | 1596 | 1550 | 257 | 189 | 182 | 70 | 69 | |||||
| 36 Kr | 14326 | 1921 | 1730,9 | 1678,4 | 292,8 | 222,2 | 214,4 | 95 | 93,8 | 27,5 | 14,1 | 14,1 | ||
| 37 Rb | 15200 | 2065 | 1864 | 1804 | 326,7 | 248,7 | 239,1 | 113 | 112 | 30,5 | 16,3 | 15,3 | ||
| 38 Sr | 16105 | 2216 | 2007 | 1940 | 358,7 | 280,3 | 270 | 136 | 134,2 | 38,9 | 21,3 | 20,1 | ||
| 39 Y | 17038 | 2373 | 2156 | 2080 | 392 | 310,6 | 298,8 | 157,7 | 155,8 | 43,8 | 24,4 | 23,1 | ||
| 40 Zr | 17998 | 2532 | 2307 | 2223 | 430,3 | 343,5 | 329,8 | 181,1 | 178,8 | 50,6 | 28,5 | 27,1 | ||
| 41 Nb | 18986 | 2698 | 2465 | 2371 | 466,6 | 376,1 | 360,6 | 205 | 202,3 | 56,4 | 32,6 | 30,8 | ||
| 42 Mo | 20000 | 2866 | 2625 | 2520 | 506,3 | 411,6 | 394 | 231,1 | 227,9 | 63,2 | 37,6 | 35,5 | ||
| 43 Tc | 21044 | 3043 | 2793 | 2677 | 544 | 447,6 | 417,7 | 257,6 | 253,9 | 69,5 | 42,3 | 39,9 | ||
| 44 Ru | 22117 | 3224 | 2967 | 2838 | 586,1 | 483,5 | 461,4 | 284,2 | 280 | 75 | 46,3 | 43,2 | ||
| 45 Rh | 23220 | 3412 | 3146 | 3004 | 628,1 | 521,3 | 496,5 | 311,9 | 307,2 | 81,4 | 50,5 | 47,3 | ||
| 46 Pd | 24350 | 3604 | 3330 | 3173 | 671,6 | 559,9 | 532,3 | 340,5 | 335,2 | 87,1 | 55,7 | 50,9 | ||
| 47 Ag | 25514 | 3806 | 3524 | 3351 | 719 | 603,8 | 573 | 374 | 368,3 | 97 | 63,7 | 58,3 | ||
| 48 Cd | 26711 | 4018 | 3727 | 3538 | 772 | 652,6 | 618,4 | 411,9 | 405,2 | 109,8 | 63,9 | 63,9 | 11,7 | 10,7 |
| 49 In | 27940 | 4238 | 3938 | 3730 | 827,2 | 703,2 | 665,3 | 451,4 | 443,9 | 122,9 | 73,5 | 73,5 | 17,7 | 16,9 |
| 50 Sn | 29200 | 4465 | 4156 | 3929 | 884,7 | 756,5 | 714,6 | 493,2 | 484,9 | 137,1 | 83,6 | 83,6 | 24,9 | 23,9 |
| 51 Sb | 30491 | 4698 | 4380 | 4132 | 946 | 812,7 | 766,4 | 537,5 | 528,2 | 153,2 | 95,6 | 95,6 | 33,3 | 32,1 |
| 52 Te | 31814 | 4939 | 4612 | 4341 | 1006 | 870,8 | 820 | 583,4 | 573 | 169,4 | 103,3 | 103,3 | 41,9 | 40,4 |
| 53 I | 33169 | 5188 | 4852 | 4557 | 1072 | 931 | 875 | 630,8 | 619,3 | 186 | 123 | 123 | 50,6 | 48,9 |
Xenon (54) to gold (79)
| Orbital | 1s | 2s | 2p1/2 | 2p3/2 | 3s | 3p1/2 | 3p3/2 | 3d3/2 | 3d5/2 | 4s | 4p1/2 | 4p3/2 | 4d3/2 | 4d5/2 | 4f5/2 | 4f7/2 | 5s | 5p1/2 | 5p3/2 |
| 54 Xe | 34561 | 5453 | 5107 | 4786 | 1148,7 | 1002,1 | 940,6 | 689 | 676,4 | 213,2 | 146,7 | 145,5 | 69,5 | 67,5 | — | — | 23,3 | 13,4 | 12,1 |
| 55 Cs | 35985 | 5714 | 5359 | 5012 | 1211 | 1071 | 1003 | 740,5 | 726,6 | 232,3 | 172,4 | 161,3 | 79,8 | 77,5 | — | — | 22,7 | 14,2 | 12,1 |
| 56 Ba | 37441 | 5989 | 5624 | 5247 | 1293 | 1137 | 1063 | 795,7 | 780,5 | 253,5 | 192 | 178,6 | 92,6 | 89,9 | — | — | 30,3 | 17 | 14,8 |
| 57 La | 38925 | 6266 | 5891 | 5483 | 1362 | 1209 | 1128 | 853 | 836 | 274,7 | 205,8 | 196 | 105,3 | 102,5 | — | — | 34,3 | 19,3 | 16,8 |
| 58 Ce | 40443 | 6549 | 6164 | 5723 | 1436 | 1274 | 1187 | 902,4 | 883,8 | 291 | 223,2 | 206,5 | 109 | — | 0,1 | 0,1 | 37,8 | 19,8 | 17 |
| 59 Pr | 41991 | 6835 | 6440 | 5964 | 1511 | 1337 | 1242 | 948,3 | 928,8 | 304,5 | 236,3 | 217,6 | 115,1 | 115,1 | 2 | 2 | 37,4 | 22,3 | 22,3 |
| 60 Nd | 43569 | 7126 | 6722 | 6208 | 1575 | 1403 | 1297 | 1003,3 | 980,4 | 319,2 | 243,3 | 224,6 | 120,5 | 120,5 | 1,5 | 1,5 | 37,5 | 21,1 | 21,1 |
| 61 Pm | 45184 | 7428 | 7013 | 6459 | 1471 | 1357 | 1052 | 1027 | 242 | 242 | 120 | 120 | — | — | — | — | — | ||
| 62 Sm | 46834 | 7737 | 7312 | 6716 | 1723 | 1541 | 1420 | 1110,9 | 1083,4 | 347,2 | 265,6 | 247,4 | 129 | 129 | 5,2 | 5,2 | 37,4 | 21,3 | 21,3 |
| 63 Eu | 48519 | 8052 | 7617 | 6977 | 1800 | 1614 | 1481 | 1158,6 | 1127,5 | 360 | 284 | 257 | 133 | 127,7 | 0 | 0 | 32 | 22 | 22 |
| 64 Gd | 50239 | 8376 | 7930 | 7243 | 1881 | 1688 | 1544 | 1221,9 | 1189,6 | 378,6 | 286 | 271 | — | 142,6 | 8,6 | 8,6 | 36 | 28 | 21 |
| 65 Tb | 51996 | 8708 | 8252 | 7514 | 1968 | 1768 | 1611 | 1276,9 | 1241,1 | 396 | 322,4 | 284,1 | 150,5 | 150,5 | 7,7 | 2,4 | 45,6 | 28,7 | 22,6 |
| 66 Dy | 53789 | 9046 | 8581 | 7790 | 2047 | 1842 | 1676 | 1333 | 1292,6 | 414,2 | 333,5 | 293,2 | 153,6 | 153,6 | 8 | 4,3 | 49,9 | 26,3 | 26,3 |
| 67 Ho | 55618 | 9394 | 8918 | 8071 | 2128 | 1923 | 1741 | 1392 | 1351 | 432,4 | 343,5 | 308,2 | 160 | 160 | 8,6 | 5,2 | 49,3 | 30,8 | 24,1 |
| 68 Er | 57486 | 9751 | 9264 | 8358 | 2207 | 2006 | 1812 | 1453 | 1409 | 449,8 | 366,2 | 320,2 | 167,6 | 167,6 | — | 4,7 | 50,6 | 31,4 | 24,7 |
| 69 Tm | 59390 | 10116 | 9617 | 8648 | 2307 | 2090 | 1885 | 1515 | 1468 | 470,9 | 385,9 | 332,6 | 175,5 | 175,5 | — | 4,6 | 54,7 | 31,8 | 25 |
| 70 Yb | 61332 | 10486 | 9978 | 8944 | 2398 | 2173 | 1950 | 1576 | 1528 | 480,5 | 388,7 | 339,7 | 191,2 | 182,4 | 2,5 | 1,3 | 52 | 30,3 | 24,1 |
| 71 Lu | 63314 | 10870 | 10349 | 9244 | 2491 | 2264 | 2024 | 1639 | 1589 | 506,8 | 412,4 | 359,2 | 206,1 | 196,3 | 8,9 | 7,5 | 57,3 | 33,6 | 26,7 |
| 72 Hf | 65351 | 11271 | 10739 | 9561 | 2601 | 2365 | 2108 | 1716 | 1662 | 538 | 438,2 | 380,7 | 220 | 211,5 | 15,9 | 14,2 | 64,2 | 38 | 29,9 |
| 73 Ta | 67416 | 11682 | 11136 | 9881 | 2708 | 2469 | 2194 | 1793 | 1735 | 563,4 | 463,4 | 400,9 | 237,9 | 226,4 | 23,5 | 21,6 | 69,7 | 42,2 | 32,7 |
| 74 W | 69525 | 12100 | 11544 | 10207 | 2820 | 2575 | 2281 | 1872 | 1809 | 594,1 | 490,4 | 423,6 | 255,9 | 243,5 | 33,6 | 31,4 | 75,6 | 45,3 | 36,8 |
| 75 Re | 71676 | 12527 | 11959 | 10535 | 2932 | 2682 | 2367 | 1949 | 1883 | 625,4 | 518,7 | 446,8 | 273,9 | 260,5 | 42,9 | 40,5 | 83 | 45,6 | 34,6 |
| 76 Os | 73871 | 12968 | 12385 | 10871 | 3049 | 2792 | 2457 | 2031 | 1960 | 658,2 | 549,1 | 470,7 | 293,1 | 278,5 | 53,4 | 50,7 | 84 | 58 | 44,5 |
| 77 Ir | 76111 | 13419 | 12824 | 11215 | 3174 | 2909 | 2551 | 2116 | 2040 | 691,1 | 577,8 | 495,8 | 311,9 | 296,3 | 63,8 | 60,8 | 95,2 | 63 | 48 |
| 78 Pt | 78395 | 13880 | 13273 | 11564 | 3296 | 3027 | 2645 | 2202 | 2122 | 725,4 | 609,1 | 519,4 | 331,6 | 314,6 | 74,5 | 71,2 | 101,7 | 65,3 | 51,7 |
| 79 Au | 80725 | 14353 | 13734 | 11919 | 3425 | 3148 | 2743 | 2291 | 2206 | 762,1 | 642,7 | 546,3 | 353,2 | 335,1 | 87,6 | 84 | 107,2 | 74,2 | 57,2 |
Mercury (80) to uranium (92)
| Orbital | 1s | 2s | 2p1/2 | 2p3/2 | 3s | 3p1/2 | 3p3/2 | 3d3/2 | 3d5/2 | 4s | 4p1/2 | 4p3/2 | 4d3/2 | 4d5/2 | 4f5/2 | 4f7/2 | 5s | 5p1/2 | 5p3/2 | 5d3/2 | 5d5/2 | 6s | 6p1/2 | 6p3/2 |
| 80 Hg | 83102 | 14839 | 14209 | 12284 | 3562 | 3279 | 2847 | 2385 | 2295 | 802,2 | 680,2 | 576,6 | 378,2 | 358,8 | 104 | 99,9 | 127 | 83,1 | 64,5 | 9,6 | 7,8 | |||
| 81 Tl | 85530 | 15347 | 14698 | 12658 | 3704 | 3416 | 2957 | 2485 | 2389 | 846,2 | 720,5 | 609,5 | 405,7 | 385 | 122,2 | 117,8 | 136 | 94,6 | 73,5 | 14,7 | 12,5 | |||
| 82 Pb | 88005 | 15861 | 15200 | 13035 | 3851 | 3554 | 3066 | 2586 | 2484 | 891,8 | 761,9 | 643,5 | 434,3 | 412,2 | 141,7 | 136,9 | 147 | 106,4 | 83,3 | 20,7 | 18,1 | |||
| 83 Bi | 90524 | 16388 | 15711 | 13419 | 3999 | 3696 | 3177 | 2688 | 2580 | 939 | 805,2 | 678,8 | 464 | 440,1 | 162,3 | 157 | 159,3 | 119 | 92,6 | 26,9 | 23,8 | |||
| 84 Po | 93105 | 16939 | 16244 | 13814 | 4149 | 3854 | 3302 | 2798 | 2683 | 995 | 851 | 705 | 500 | 473 | 184 | 184 | 177 | 132 | 104 | 31 | 31 | |||
| 85 At | 95730 | 17493 | 16785 | 14214 | 4317 | 4008 | 3426 | 2909 | 2787 | 1042 | 886 | 740 | 533 | 507 | 210 | 210 | 195 | 148 | 115 | 40 | 40 | |||
| 86 Rn | 98404 | 18049 | 17337 | 14619 | 4482 | 4159 | 3538 | 3022 | 2892 | 1097 | 929 | 768 | 567 | 541 | 238 | 238 | 214 | 164 | 127 | 48 | 48 | 26 | ||
| 87 Fr | 101137 | 18639 | 17907 | 15031 | 4652 | 4327 | 3663 | 3136 | 3000 | 1153 | 980 | 810 | 603 | 577 | 268 | 268 | 234 | 182 | 140 | 58 | 58 | 34 | 15 | 15 |
| 88 Ra | 103922 | 19237 | 18484 | 15444 | 4822 | 4490 | 3792 | 3248 | 3105 | 1208 | 1058 | 879 | 636 | 603 | 299 | 299 | 254 | 200 | 153 | 68 | 68 | 44 | 19 | 19 |
| 89 Ac | 106755 | 19840 | 19083 | 15871 | 5002 | 4656 | 3909 | 3370 | 3219 | 1269 | 1080 | 890 | 675 | 639 | 319 | 319 | 272 | 215 | 167 | 80 | 80 | — | — | - |
| 90 Th | 109651 | 20472 | 19693 | 16300 | 5182 | 4830 | 4046 | 3491 | 3332 | 1330 | 1168 | 966,4 | 712,1 | 675,2 | 342,4 | 333,1 | 290 | 229 | 182 | 92,5 | 85,4 | 41,4 | 24,5 | 16,6 |
| 91 Pa | 112601 | 21105 | 20314 | 16733 | 5367 | 5001 | 4174 | 3611 | 3442 | 1387 | 1224 | 1007 | 743 | 708 | 371 | 360 | 310 | 232 | 232 | 94 | 94 | — | — | - |
| 92 U | 115606 | 21757 | 20948 | 17166 | 5548 | 5182 | 4303 | 3728 | 3552 | 1439 | 1271 | 1043 | 778,3 | 736,2 | 388,2 | 377,4 | 321 | 257 | 192 | 102,8 | 94,2 | 43,9 | 26,8 | 16,8 |