Specific Process Knowledge/Characterization/XPS/Processing/ALDSandwich1/3scanned
Processing of high resolution scanned spectra
The analysis is continued from here.
It is usually a good idea to have a look at XPS Knowledge view of each element before the analysis of the high resolution spectra.
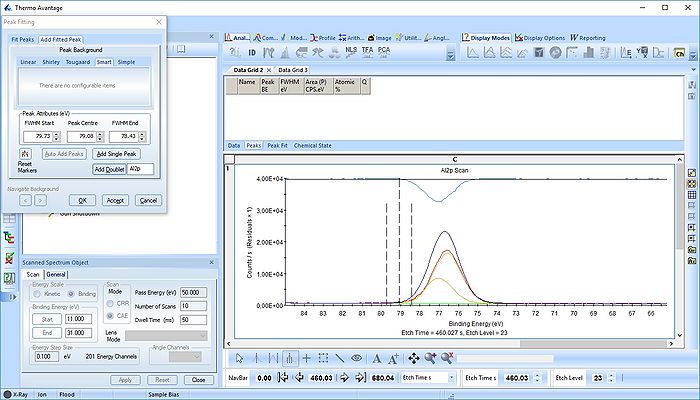
The Al2p peak
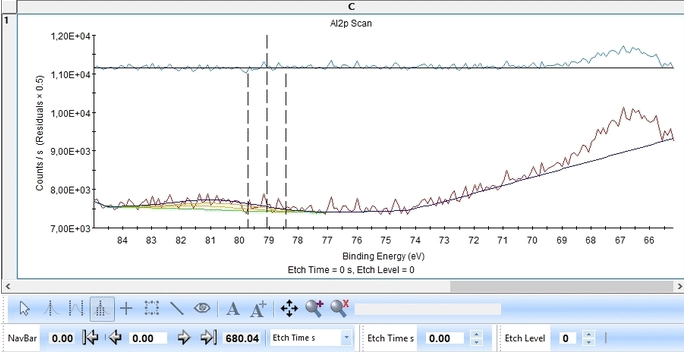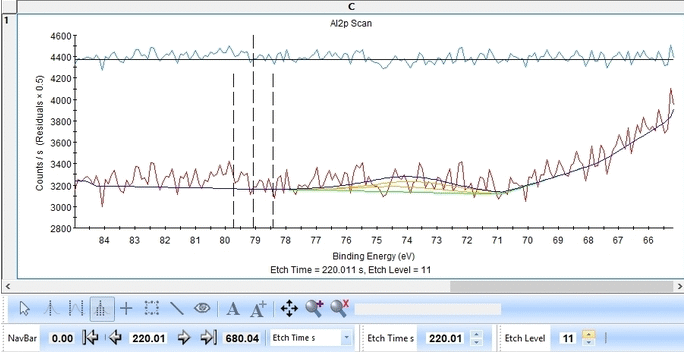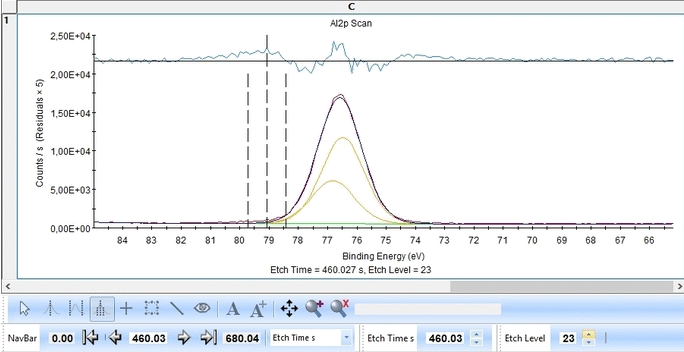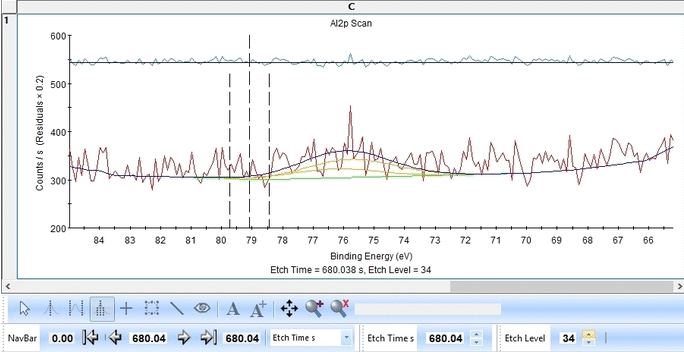
We start with the Al2p scan. Below is shown the etch level 23. Click 'Peak Fit' and select 'Add doublet' (Not 'Add Single Peak' ). In the tab 'Fit Peaks' of the 'Peak Fitting' panel click 'Fit All Levels'.
So far we did not add any constraints at all. That means the Al2p spin orbit doublet is free to move anywhere to find a minimum error.
Scrolling through the etch levels (seen in the bottom of the image below slightly to the right) one can see that the fitting is good. There is some energy shifts around etch levels 22-24 but we will ignore that for now. Whether caused by actual chemical shifts (the appearance of intermediate oxidation states of Al2p that causes the spectrum to shift) or poor energy calibration is too soon to say. The O1s peak will probably hold some clues about this.
The Hf4f peak
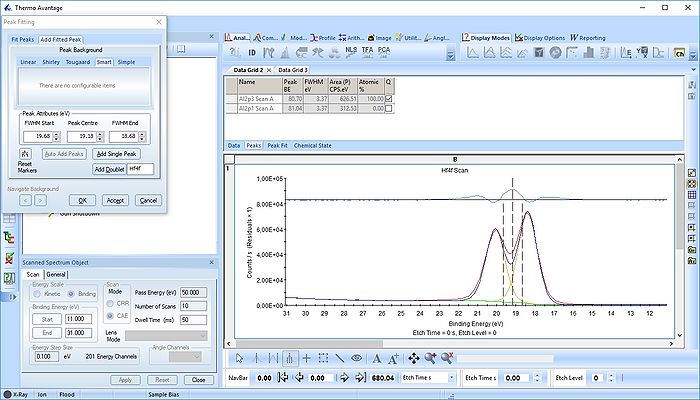
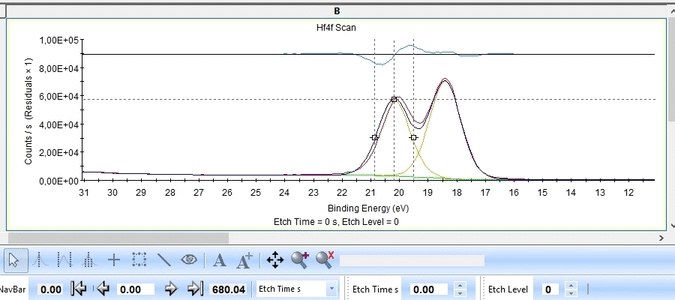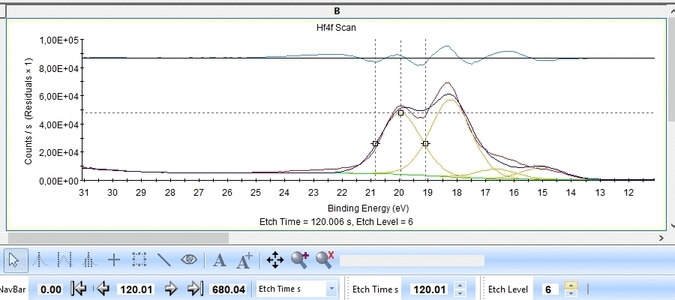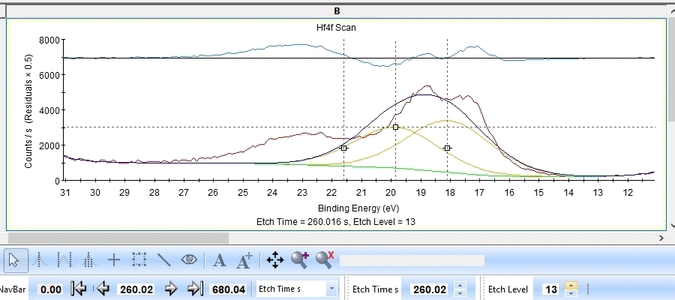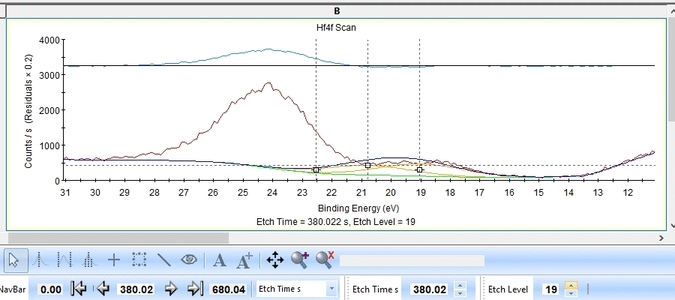
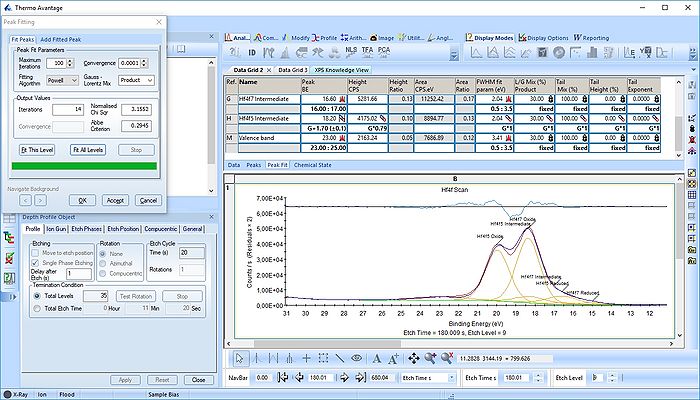
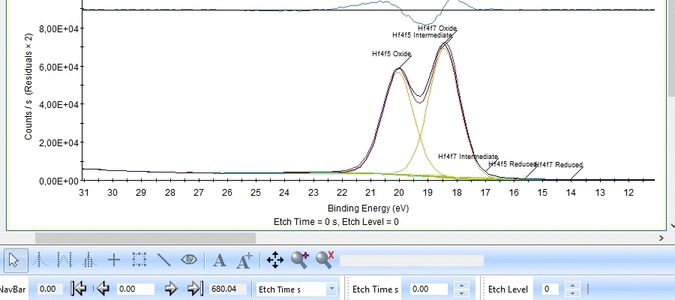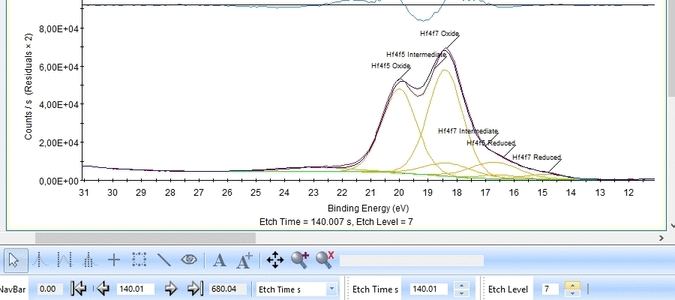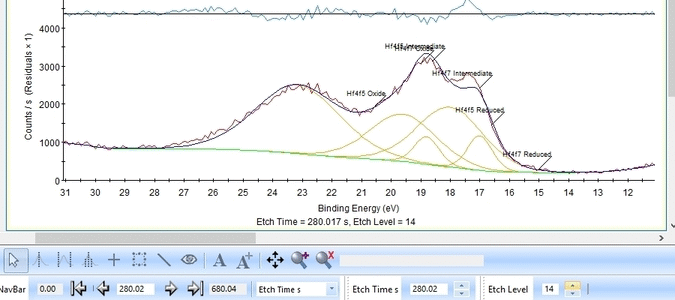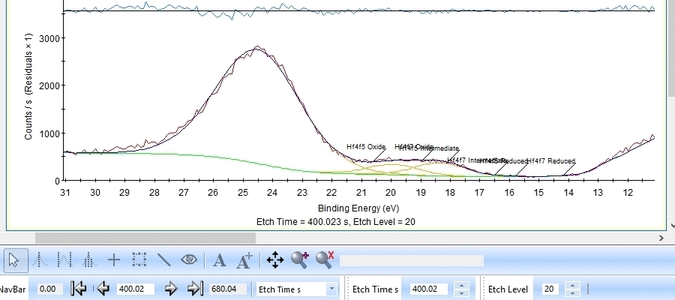
The Hf4f peak is fitted next. Maximize the peak and click 'Peak Fit'.
Etch level 0 shown above only contains the oxidized Hf4f peak - it does not have any information on the location of any reduced states. Therefore scroll to etch level 2 and add two doublets. With two or more peaks in the fitting routine we have to add some constraints on the peaks in order to maintain order and physics.
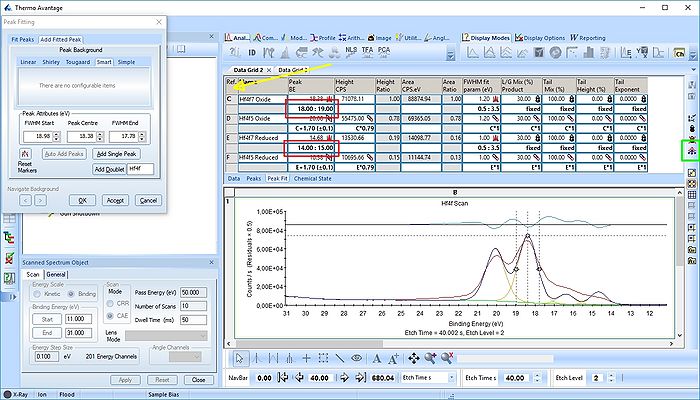
In image above the following has been done:
- Two Hf4f doublets are added and labelled 'Oxide' and 'Reduced'. Both peaks of each spin-orbit couple are renamed.
- Two constraints are added on the binding energies as indicated with red boxes: The binding energy of Hf4f7 oxide peak is constrained within the [18-19 eV] range and the reduced Hf4f7 peak is constrained within [14-15 eV].
- In order to apply the binding energy constraints and the changes in peak names to all etch levels, we first select the entire peak fit table by pressing the upper left corner of the table labelled 'Ref.' (indicated by the yellow arrow).
- With the entire peak table highlighted press the 'Propagate constraints and values to all levels' to the right of the table (indicated by a green square).
With the constraints and names applied to all etch levels, we can start the fitting routine by pressing 'Fit All Levels'. Below is a gif image of the fitting of the etch levels in which Hf can be expected to be present.
We note the following:
- The Hf4f peak is at very low binding energy, very near or even in the valence band. We can therefore expect to see contributions from other elements as well. This begins at etch level 10 or 11 where the intensity from Hf4f is low. Most notably, a peak at binding energy 23-24 eV becomes dominant beyond etch level 13. It would probably make most sense to add a peak constrained in this binding energy range and then exclude that peak from the subsequent quantifications.
- At etch levels 1 to 9 the fitting routine is struggling to fit the intensity at binding energies around 16 and 18 eV. This points strongly towards the presence of intermediate oxidation steps.
We add a third Hf4f doublet and label it 'Intermediate' - this is also suggested in The XPS Knowledge view for hafnium. A single peak is also added and labelled 'Valence', see below. The binding energies of the peaks are constrained as shown below.
The fitting is much better as seen below. One could probably argue that the presence of the Valence band peak is irrelevant as it only becomes important after etch level 9-11 where the Hf is essentially gone.
The peak fitting of the Hf4f peak is completed by pressing the 'Accept' button.
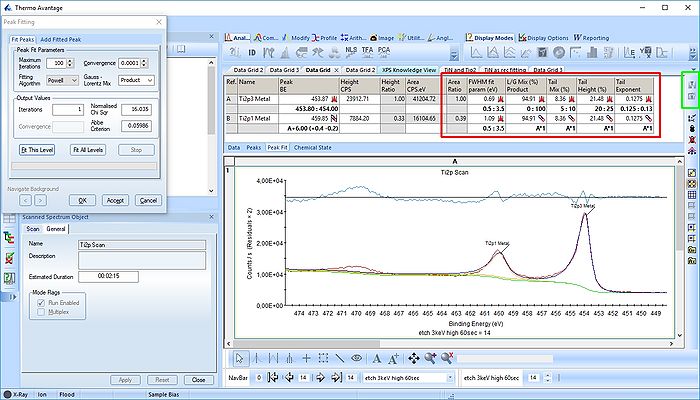
The Ti2p peak
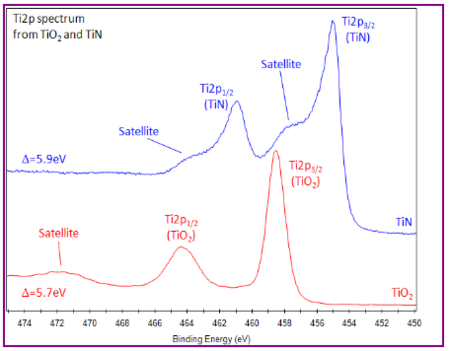
The titanium Ti2p peak is notoriously difficult to fit. From XPS Knowledge view we have:
- Ti metal gives asymmetric Ti2p peak shapes
- TiO2 has symmetric peak shapes and TiN has complex peak shape, involving satellite features.
- Ti2p peak has significantly split spin-orbit components (Δmetal=6.1 eV)
- Splitting Δ-value varies with chemical state (Δnitride=6.0 eV,Δoxide=5.7 eV).
- Typically FWHM for each spin-orbit component is the same, but for Ti2p the Ti2p1/2 component is much broader than the Ti2p3/2 peak.
- Consequently, Ti2p1/2 peak is much shorter than expected.
- Caused by Coster-Kronig effect. (Post-ionization, Ti2p1/2 is very short lived compared to Ti2p3/2 state.)
- Causes difficulty in accurately peak fitting Ti2p region with multiple chemical states.''
General comments
- Metal is readily oxidized.
- Titanium is used as a getter material for oxygen.
- TiO2 is readily reduced by argon monomer sputtering
- Sub-oxides and/or metal may be formed during sputtering of the oxide.
Please note that clicking on any of the two spectra above when using XPS Knowledge View will generate a new processing grid in Avantage with this particular set of data, peak table etc. It may be quite convenient to investigate what constraints have been added - obviously also for any other element.
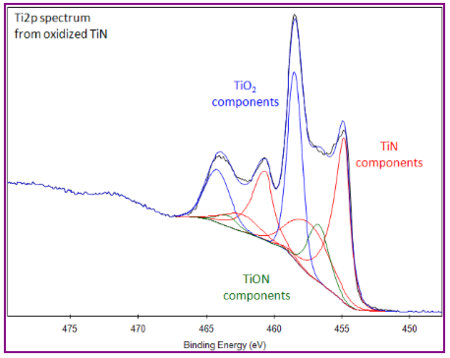
The only challenge we are missing from this cocktail of asymmetric peak shapes, differences in spin-orbit splitting, reduction of fully oxidized Ti2p to sub-oxides and satellite features is the presence of TiN.
First, let us find some reasonable parameters for the Ti2p metallic peak. For that purpose, we analyse an old data set acquired on pure Ti.