Specific Process Knowledge/Thin film deposition/ALD Picosun R200/AZO deposition using ALD
AZO can be deposited in a temperature window 150 - 250 oC. Physical and optical properties are strongly corelated with deposition temperture and Al doping concentration. All results shown on this page have been obtained using the Si(100) wafers with native oxide as substrates:
Al-doped ZnO (AZO) deposition overview
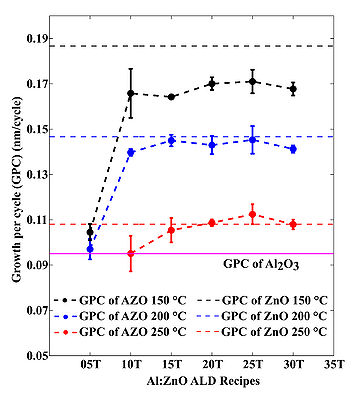
The deposition rate for AZO depends on the temperature and doping concentration, see figures that illustrate AZO growth below. The uniformity, thickness, refractive index has been obtained using Ellipsometer VASE.
-
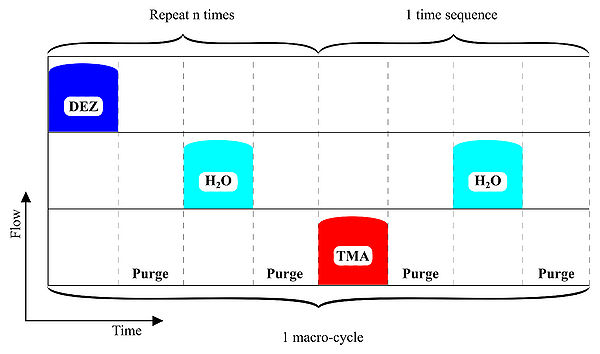
ALD-schematics for AZO deposition.
-
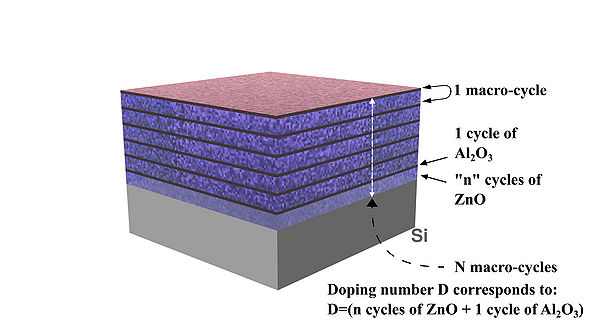
Schematic drawing of deposited AZO layers and concept illustration of doping "D" number/level 05, 10, 15, 20,etc.
Evgeniy Shkondin, DTU Danchip, 2015-2016.
Al-doped ZnO (AZO) standard recipes
Recipe: AZO 20T
(Number 20 means doping level "D": 19 cycles of ZnO + 1 cycle Al2O3, see details in the table below)
Maximum deposition thickness: 100 nm
Temperature: 150 oC - 250 oC
| # macrocycles | N | |||||||
|---|---|---|---|---|---|---|---|---|
| # cycles | 19 | 1 | ||||||
| Precursor | DEZ | DEZ | H2O | H2O | TMA | TMA | H2O | H2O |
| Nitrogen flow | 150 sccm | 150 sccm | 200 sccm | 200 sccm | 150 sccm | 150 sccm | 200 sccm | 200 sccm |
| Pulse time | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s |
| Purge time | 0.5 s | 20.0 s | 0.5 s | 20.0 s | 0.5 s | 20.0 s | 0.5 s | 20.0 s |
How to fill out the process log in LabManager:
The pulse time for each precursor equals the total pulse time times the number of cycles in each macrocycle.
DEZ pulse time: (0.1 + 0.1) s * 19 = 3.8 s
TMA pulse time: (0.1 + 0.1 )s * 1 = 0.2 s
H2O pulse time: [(0.1 + 0.1) s * 19] + [(0.1 + 0.1) s * 1] = 4.0 s
The number of cycles for each precursor now equals the number of macrocycles (n).
Recipe: AZO 25T
Number 25 means doping level "D": 14 cycles of ZnO + 1 cycle Al2O3, see details in the table below)
Maximum deposition thickness: 100 nm
Temperature: 150 oC - 250 oC
| # macrocycles | N | |||||||
|---|---|---|---|---|---|---|---|---|
| # cycles | 24 | 1 | ||||||
| Precursor | DEZ | DEZ | H2O | H2O | TMA | TMA | H2O | H2O |
| Nitrogen flow | 150 sccm | 150 sccm | 200 sccm | 200 sccm | 150 sccm | 150 sccm | 200 sccm | 200 sccm |
| Pulse time | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s |
| Purge time | 0.5 s | 20.0 s | 0.5 s | 20.0 s | 0.5 s | 20.0 s | 0.5 s | 20.0 s |
How to fill out the process log in LabManager:
The pulse time for each precursor equals the total pulse time times the number of cycles in each macrocycle.
DEZ pulse time: (0.1 + 0.1) s * 24 = 4.8 s
TMA pulse time: (0.1 + 0.1 )s * 1 = 0.2 s
H2O pulse time: [(0.1 + 0.1) s * 24] + [(0.1 + 0.1) s * 1] = 5.0 s
The number of cycles for each precursor now equals the number of macrocycles (n).
Recipe: AZO 30T
(Number 30 means doping level "D": 29 cycles of ZnO + 1 cycle Al2O3, see details in the table below)
Maximum deposition thickness: 100 nm
Temperature: 150 oC - 250 oC
| # macrocycles | N | |||||||
|---|---|---|---|---|---|---|---|---|
| # cycles | 29 | 1 | ||||||
| Precursor | DEZ | DEZ | H2O | H2O | TMA | TMA | H2O | H2O |
| Nitrogen flow | 150 sccm | 150 sccm | 200 sccm | 200 sccm | 150 sccm | 150 sccm | 200 sccm | 200 sccm |
| Pulse time | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s |
| Purge time | 0.5 s | 20.0 s | 0.5 s | 20.0 s | 0.5 s | 20.0 s | 0.5 s | 20.0 s |
How to fill out the process log in LabManager:
The pulse time for each precursor equals the total pulse time times the number of cycles in each macrocycle.
DEZ pulse time: (0.1 + 0.1) s * 29 = 5.8 s
TMA pulse time: (0.1 + 0.1 )s * 1 = 0.2 s
H2O pulse time: [(0.1 + 0.1) s * 29] + [(0.1 + 0.1) s * 1] = 6.0 s
The number of cycles for each precursor now equals the number of macrocycles (n).
Recipe: AZO 35T
(Number 35 means doping level "D": 34 cycles of ZnO + 1 cycle Al2O3, see details in the table below)
Maximum deposition thickness: 100 nm
Temperature: 150 oC - 250 oC
| # macrocycles | N | |||||||
|---|---|---|---|---|---|---|---|---|
| # cycles | 34 | 1 | ||||||
| Precursor | DEZ | DEZ | H2O | H2O | TMA | TMA | H2O | H2O |
| Nitrogen flow | 150 sccm | 150 sccm | 200 sccm | 200 sccm | 150 sccm | 150 sccm | 200 sccm | 200 sccm |
| Pulse time | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s | 0.1 s |
| Purge time | 0.5 s | 20.0 s | 0.5 s | 20.0 s | 0.5 s | 20.0 s | 0.5 s | 20.0 s |
How to fill out the process log in LabManager:
The pulse time for each precursor equals the total pulse time times the number of cycles in each macrocycle.
DEZ pulse time: (0.1 + 0.1) s * 34 = 6.8 s
TMA pulse time: (0.1 + 0.1 )s * 1 = 0.2 s
H2O pulse time: [(0.1 + 0.1) s * 34] + [(0.1 + 0.1) s * 1] = 7.0 s
The number of cycles for each precursor now equals the number of macrocycles (n).
XPS investigation of AZO thin films
XPS profiles for AZO has been obtained using XPS-ThermoScientific equipment.
- XPS Investigation of AZO layers.
-
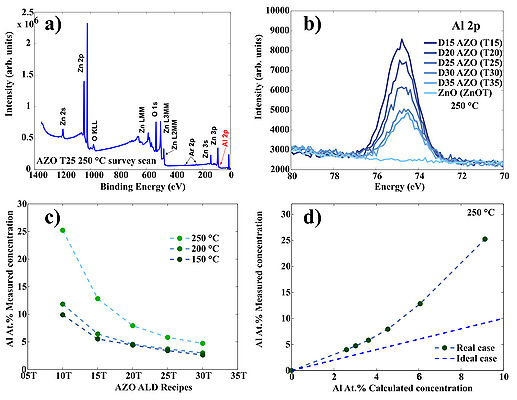
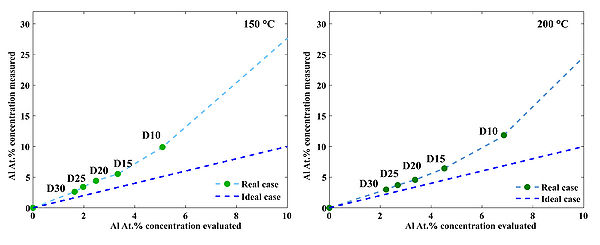
Typical survey scan of AZO/ZnO. b) High resolution scan of the Al 2p region for AZO/ZnO samples prepared at 250 oC c) Measured Al cocncentration in AZO films prepared at three temperatures: 150 oC, 200 oC and 250 oC. d) Deviation of the measured Al at.% concentaration from the theoretical estimation in case of 250 oC deposition temperature. Theoretical estimation can be calculated using following formula: , where GPCAl2O3 and GPCZnO are growth-per-cycle of Al2O3 and ZnO at given deposition temperature, respectively. "n" is a doping level: 05, 10, 15, 20 etc.
-
Deviation of the measured Al at.% concentaration from the theoretical estimation in case of 150 oC and 200 oC deposition temperature. Deviations occure due to nucleation issues of ZnO on top of TMA-H2O passiveted surfaces.
| Recipes | 150 oC. Al at.% Concentration mesured using (XPS) | 200 oC. Al at.% Concentration mesured using (XPS) | 250 oC. Al at.% Concentration mesured using (XPS) |
|---|---|---|---|
| ZnOT (no doping) | 0 | 0 | 0 |
| AZO 05T | 30.45 | not measured | not measured |
| AZO 10T | 9.89 | 11.84 | 25.23 |
| AZO 15T | 5.52 | 6.16 | 12.82 |
| AZO 20T | 4.41 | 4.35 | 7.93 |
| AZO 25T | 3.41 | 3.61 | 5.82 |
| AZO 30T | 2.62 | 3.18 | 4.73 |
| AZO 35T | not measured | not measured | 3.98 |
Evgeniy Shkondin, DTU Danchip, 2015-2016.
Morphology of deposited AZO layers
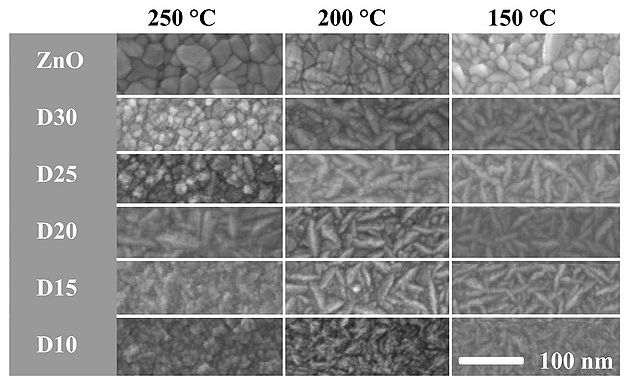
The surface morphology of deposited 100 nm ZnO/AZO thin films has been analyzed by scanning electron microscopy and atomic force microscopy.
SEM morphology
AFM morphology
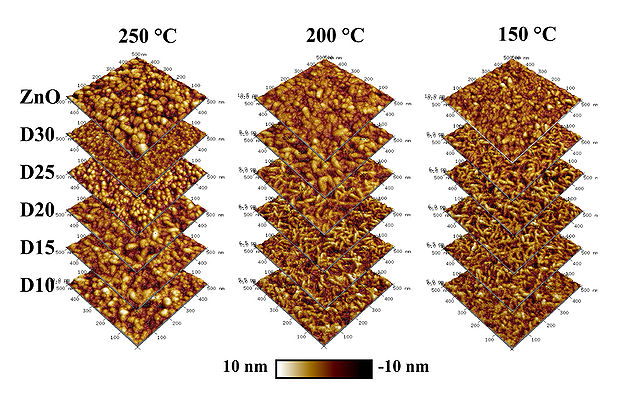
AFM inspections of deposited ZnO/AZO films D10-D35 at 250 oC reveals a surface roughness of approx. 2 nm RMS, which slightly decreases with increasing Al content in the films. In case of D10, the Al2O3 phase dominates and it result in a roughness below the acceptable detection value. The AZO films deposited at 250 oC tend to obtain a cylindrical morphology, which can be related to preferred (002) ZnO crystal orientation growth.
Evgeniy Shkondin, DTU Danchip, 2015-2016.
X-ray diffraction measurements
The crystal orientations have been studied by analyzing X-ray diffraction (XRD) patters. The equipment is D8 diffractometer from Bruker located at DTU Mechanical Engineering Department. θ -2\θ scans were acquired in a Bragg-Brentano geometry with a Cu Kα X-ray source. The instrumental broadening was taken into account by performing a LaB6 powder standard scan. The obtained scans were additionally checked for the alignment by controlling the corresponding position of the Si(400) K\α1 and Kβ peaks coming from the Si substrates.
XRD results are summarized in the figure below. It is revealed that AZO films deposited at 250 oC are polycrystalline films with preferential (002) orientation. Two main peaks have been found: (002) and weak (101), all other orientations were not observed in the range 2θ between 20 and 72o. Figure (e) below shows, ZnO/AZO XRD obtained patterns at 250 oC.
A (100) peak is observed in the 150 oC deposited films, where this orientation is preferential. In 150 oC AZO films both (100) and (002) peaks are present. XRD results obtained at 150 oC and 200 oC are shown in Figure (a) and (c). Finally, the (100) orientation vanished at 250 oC and is not observed in Figure (e). The noisy part from quasi-forbidden Si (200) reflections has been removed from the scans.
The central positions of the orientation peaks move to higher 2θ values with increasing of Al concentrations in the films. When the concentration becomes too high (like in the case of D15 and D10 recipes processed at 250 oC), the films become amorphous (see Figure (a)). Characterization of the crystal structure is conducted by fitting the Lorentzian functions through the (002) and (101) peaks ( see Figure (a)). According to the Bragg law: