Specific Process Knowledge/Characterization/Element analysis
Feedback to this page: click here
Element analysis at Danchip
The following techniques for elemental analysis are available at Danchip.
- EDX
- SIMS
- XPS (ESCA)
In the table below the three techniques are compared
Comparison of EDX, SIMS and XPS
| SEM with EDX | Atomika SIMS | XPS (or ESCA) | |
|---|---|---|---|
| Full name | Energy Dispersive X-ray Analysis | Secondary Ion Mass Spectroscopy | X-ray Photoelectron Spectroscopy (or Electron Spectroscopy for Chemical Analysis) |
| Technique | The primary beam of high energy electrons used in the SEM for imaging impinges on the sample atoms and leaves them in an excited state. X-rays with a characteristic energy are generated in the relaxation process. The combination of the fine control of the primary beam offered by the SEM and the detection of the X-rays makes it possible to make point-like elemental analysis. | A beam of high energy ions (cesium or oxygen) is used for sputtering off surface atoms of the sample. The material coming off the sample in this process is analysed with a mass spectrometer. | In a process in which a monochromatic beam of X-rays irradiates the sample surface, electrons bound inside the sample are knocked free to become photoelectrons. Escaping the sample with characteristic energy, these electrons not only carry elemental information but also chemical information. Analysing them respect to energy and numbers and adding an ion gun for depth profiles provide a powerful analysis tool. |
| What elements are detected | Every element heavier than boron/carbon | In principle every element, however, the sensitivity | Every element except hydrogen and helium. |
| Chemical information | None | None | Chemical state information |
| Sample requirements | Vacuum compatible |
|
|
| Spatial resolution | Very precise point-like analysis is possible with SEM electron beam. | A square with dimensions of a few hundred microns is selected for analyis with a camera | Using a magnified view from a camera, a point that covers an area down to an ellipse of 40 microns may be irradiated with photons (the default size is 400 microns) |
| Depth resolution | The size of the interaction volume depends on the high voltage in the SEM and the sample density: The higher the SEM high voltage the bigger and deeper the interaction volume. The more dense the material is the smaller is the interaction volume. See section 'Spatial resolution using EDX' below. | The sputtering of the surface makes it possible to perform detailed depth profiling with extremely good sensitivity and depth resolution. | Very surface sensitive technique. Only photoelectrons from the top layer (a few nanometers deep) escape unscattered. By using the ion beam etch, the composition of deeper lying layers can be probed. |
| Detection limit | Approximately 1 % atomic weight | Down to 1 ppb for certain elements | Approximately 1 % atomic weight |
| Speed of measurement | Quite fast and easy | Time consuming | Quite fast and easy |
- The techniques EDX, SIMS and XPS
-
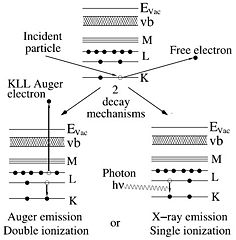
The high energy electrons in the beam (denoted above as incident particle) collide with the core electrons of the sample atoms that are left in an excited state with higher energy. One decay mechanism is to let an outer electron fall into the unoccupied state under emission of a photon that carries the excess energy. This energy is determined by the electronic shells and hence characteristic of the atom.
-
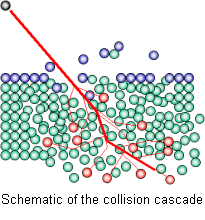
A beam of high energy ions is rastered on the surface of the sample. Some of the atoms that used to make up the surface are sputtered off and emitted as secondary ions. The mass of the these ions is measured with a mass spectrometer.
-
The atoms in the sample is irradiated with X-rays and the energy of the incoming photons are adsorbed. Photoelectrons are ejected, and the energy of these electrons can be measured. Since the energy of the incoming photon is known, the binding energy of the electrons in the atom can be determined. The binding energy is characteristic for the element, and therefore the composition of the sample can be determined.
Secondary Ion Mass Spectrometry (SIMS)
In the Atomika SIMS the samples are bombarded with a beam of either oxygen or caesium ions. When accelerated to high energy and rastered across the sample these ions will be able to gradually sputter off the surface atoms in a small area defined by the raster pattern. Some of the surface atoms are emitted as ionized particles. In this way one layer after another is peeled off the sample.
These charged species are led through a mass spectrometer where a magnetic field is used to deflect them. The deflection increases with charge and decreases with mass and we are therefore able detect and count them according to their mass. This technique is called Secondary Ion Mass Spectrometry or SIMS.
Typical application of SIMS
SIMS is the most sensitive technique for elemental composition. It is therefore ideal if you want to check doping profiles or for contaminations.
A typical application would be to check the concentration profile of boron doping in silicon. In that case one would put two samples into the SIMS.
- A reference sample with a known boron profile
- A sample
X-ray Photoelectron Spectroscopy analysis (XPS)
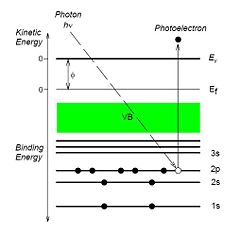
During a XPS (X-ray Photoelectron Spectroscopy) analysis, the sample is irradiated with photons of a specific energy (in the Danchip system 1486 eV). When energy of the irradiating X-rays is adsorbed by the atoms in the sample, photoelectrons are ejected [[1]].
Since the energy of the incoming photons is known, and the energy of the ejected electrons is measured, the binding energy of the electrons in the probed atoms can be determined. The binding energy of the electrons are element specific, and is therefore a "finger-print" of the atom. Hence, a measurement of the XPS spectrum gives information of which materials are present in the sample, and at which concentrations.
XPS is an excellent technique to probe the chemical state of atoms on a surface. The binding energy of lower lying atomic levels (for example 1s, 2s and 2p) are at a specific energy, but is slightly affected by the chemical environment of the probed atom. This is known as the chemical shift. By measuring the shift of the electron binding energies one can determined the chemical state of atoms. See an example on the page XPS-ThermoScientific.
Typical applications of XPS
The XPS can be used for different applications, for example:
- Do an elemental analysis of the outermost layer of your surface.
- Check the composition of a film at different depths.
- Check for a contaminations.
- It not as sensitive as the SIMS, but faster, so it can be an alternative if you are checking for a bit higher contamination levels (like 1 %)
- Do a analysis of the chemical state of atoms present on the surface.
- See what effect a surface treatment of your sample has on the surface chemistry.
- Check a polymer covered surface. Are for example (C=O), (C-OH) (C-C) groups present in the polymer after it been deposited on a surface.