Specific Process Knowledge/Thin film deposition/Antistiction Coating: Difference between revisions
Created page with "== Processing on the MVD == The MVD coatings are created as self-assembled monolayers on a surface when a molecular vapor of chemials is present. These chemicals, typically..." |
|||
| Line 5: | Line 5: | ||
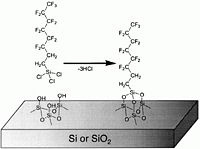
These chemicals, typically flourinated organosilanes, have a teflon-like tail consisting of -(CF<sub>2</sub>)<sub>x</sub>CF<sub>3</sub> and, in the other end, a reactive group -Si<sub>(teflon)</sub>Cl<sub>x</sub>. As shown in the figure below, the chlorine atoms react with Si<sub>(surface)</sub>-OH groups of the surface to form a chemical bond -Si(<sub>(teflon)</sub>)-O-Si<sub>(surface)</sub>- under elimination of HCL. This means that both Si and SiO<sub>2</sub> surfaces are coated because of the native oxide on Si surfaces. | These chemicals, typically flourinated organosilanes, have a teflon-like tail consisting of -(CF<sub>2</sub>)<sub>x</sub>CF<sub>3</sub> and, in the other end, a reactive group -Si<sub>(teflon)</sub>Cl<sub>x</sub>. As shown in the figure below, the chlorine atoms react with Si<sub>(surface)</sub>-OH groups of the surface to form a chemical bond -Si(<sub>(teflon)</sub>)-O-Si<sub>(surface)</sub>- under elimination of HCL. This means that both Si and SiO<sub>2</sub> surfaces are coated because of the native oxide on Si surfaces. | ||
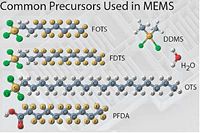
<gallery caption="Some chemicals | <gallery caption="Some chemicals used in MVD and the Si surface reaction" widths="200px" heights="150px" perrow="2"> | ||
image:chlorosilanes.jpg|Different chemicals for the MVD. | image:chlorosilanes.jpg|Different chemicals for the MVD. | ||
image:MVDsurfacereaction.jpg|The chemical reaction in which the Cl atoms of the precursors are eliminated under formation of HCl. | image:MVDsurfacereaction.jpg|The chemical reaction in which the Cl atoms of the precursors are eliminated under formation of HCl. | ||
Revision as of 09:47, 20 January 2014
Processing on the MVD
The MVD coatings are created as self-assembled monolayers on a surface when a molecular vapor of chemials is present.
These chemicals, typically flourinated organosilanes, have a teflon-like tail consisting of -(CF2)xCF3 and, in the other end, a reactive group -Si(teflon)Clx. As shown in the figure below, the chlorine atoms react with Si(surface)-OH groups of the surface to form a chemical bond -Si((teflon))-O-Si(surface)- under elimination of HCL. This means that both Si and SiO2 surfaces are coated because of the native oxide on Si surfaces.
- Some chemicals used in MVD and the Si surface reaction
-
Different chemicals for the MVD.
-
The chemical reaction in which the Cl atoms of the precursors are eliminated under formation of HCl.
The parameters of the MVD process
The most important parameters to control during the MVD process are:
- Substrate surface
- An O2 plasma is run prior to any process in order to condition the substrate surface. This will also remove any existing MVD coating.
- Water content
- Water will cause the chemicals to polymerize (bond to each other instead of on the surface) and it is therefore critical to precisely control the water content.
- Substrate temperature
- The temperature of the substrate is the same as the process chamber and it is kept constant at 35 oC.
- Vapor order
- There is no active force that injects the chemicals into the process chamber. The driving force is a combination of two factors:
- A pressure gradient The vapor pressure of the chemical (when stored at some temperature in the cylinder) compared to the pressure in the chamber.
- A temperature gradient The chemical and the line that feeds the process chamber are kept at a higher temperature.
It is critical that the chemicals with the lowest vapor pressure are injected first into the process chamber. If, for instance, water is injected before FDTS, the water will flow into the FTDS expansion volume and cause polymerization, thus forcing a mechanical clean. NOT GOOD!.
The FLAT recipe
Purpose
The FLAT recipe is designed for quick coating of non-structured wafers. One should not expect to get good conformal coverage of nanostructures or nanometersized surface roughness.
Process description
The amount of FDTS is delivered in one cycle (4 injections of FDTS at 0.400 Torr + 1 injection of water at 18 Torr) and the reaction time is 15 minutes. The total process time is some 22 minutes.
Process sequence
| O2 plasma | Flow | 200 sccm |
|---|---|---|
| Power | 250 Watts | |
| Time | 300 seconds | |
| Chemical # 1 (vapor order 1) | Name | FDTS |
| Line no. | 3 | |
| Cycles | 4 | |
| Pressure | 0.500 Torr | |
| Chemical # 2 (vapor order 2) | Name | Water |
| Line no. | 1 | |
| Cycles | 1 | |
| Pressure | 18 Torr | |
| Processing | Time | 900 seconds |
| Purge | Cycles | 5 |
The STAMP recipe
Purpose
The STAMP recipe uses the same total amount of FDTS as FLAT but delivers it much slower in order to obtain a much better and conformal coating of fine nanostructures. STAMP should always be used for stamps for nanoimprint lithography irregardless of the feature size.
Process description
A cycle with 1 injection of FDTS at 0.400 Torr + 1 injection of water at 6 Torr reacts for 15 minutes. Then the process chamber is evacuated and a new cycle starts until 4 cycles are completed. This gives a total process time of some 80 minutes.
Process sequence
| O2 plasma | Flow | 200 sccm | |
|---|---|---|---|
| Power | 250 Watts | ||
| Time | 300 seconds | ||
| Repeated 4 times | Chemical # 1 (vapor order 1) | Name | FDTS |
| Line no. | 3 | ||
| Cycles | 4 | ||
| Pressure | 0.500 Torr | ||
| Chemical # 2 (vapor order 2) | Name | Water | |
| Line no. | 1 | ||
| Cycles | 1 | ||
| Pressure | 18 Torr | ||
| Processing | Time | 900 seconds | |
| Purge | Cycles | 5 | |