Specific Process Knowledge/Characterization/XPS/XPS Chemical states: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Feedback to this page''': '''[mailto:labadviser@ | '''Feedback to this page''': '''[mailto:labadviser@nanolab.dtu.dk?Subject=Feed%20back%20from%20page%20http://labadviser.nanolab.dtu.dk/index.php?title=Specific_Process_Knowledge/Characterization/XPS/XPS_Chemical_states click here]''' | ||
{{Contentbydryetch}} | |||
=Chemical state= | =Chemical state= | ||
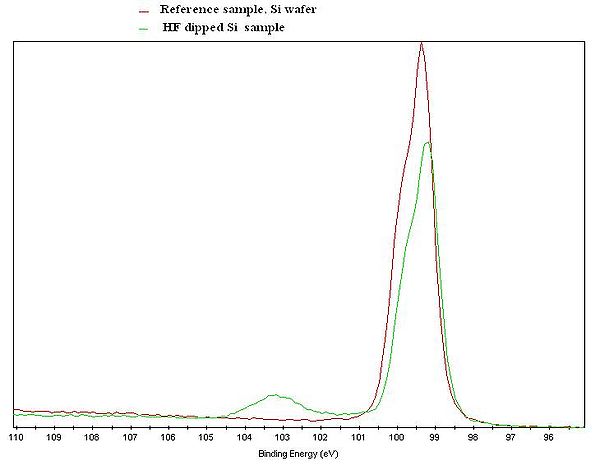
[[image:Si2p.JPG|600x600px|left|thumb|XPS Si2p spectrum of a Si reference sample (red curve), and a Si sample that was treated in HF shortly before the measurement was done (green curve). ]] | [[image:Si2p.JPG|600x600px|left|thumb|XPS Si2p spectrum of a Si reference sample (red curve), and a Si sample that was treated in HF shortly before the measurement was done (green curve). ]] | ||
From basic chemistry we know that the chemical properties of an element is determined by the outermost electronic shells. When a chemical bond between two atoms is formed there is an exchange of loosely bonded electrons between them. This exchange of valence electrons induces a small energy shift in all electronic shells of the atoms that may be detected by XPS. This is called the chemical shift. Hence, one can see if, for instance, silicon is in oxidation state 0 (Si-Si bond in bulk silicon) or oxidation state +IV (Four Si-0 bonds in SiO<sub>2</sub>) by looking at the binding energy of the core level electrons. | |||
The figure to the left | The figure to the left illustrates the chemical shift. Si2p XPS spectra were recorded an a Si reference sample and a Si sample that was treated in HF prior to the measurement - and the spectra are clearly different. The spectrum of untreated Si has a clear feature at about 103 eV due to Si atoms bonded to oxygen in the native oxide. In the spectrum from the HF treated sample, only the feature arising from Si-Si interaction is present. Note that both curves only shows the Si signal, but with an clear indication of the chemical state of the Si atoms in the samples. | ||
If you study polymers, you can detect the presence of different chemical groups, for example (C-C),(C-OH),(C=O),(CF3) or ( | If you study polymers, you can detect the presence of different chemical groups, for example (C-C),(C-OH),(C=O),(CF3) or (CF<sub>2</sub>-CH<sub>2</sub>) in the polymeric layer. And after surface treatments, you may examine differences in the polymeric layer. | ||
Note that binding energies for different chemical states often can be found in the literature. | Note that binding energies for different chemical states often can be found in the literature. | ||
Latest revision as of 11:12, 28 June 2023
Feedback to this page: click here
Unless otherwise stated, the content of this page was created by the dry etch group at DTU Nanolab
Chemical state
From basic chemistry we know that the chemical properties of an element is determined by the outermost electronic shells. When a chemical bond between two atoms is formed there is an exchange of loosely bonded electrons between them. This exchange of valence electrons induces a small energy shift in all electronic shells of the atoms that may be detected by XPS. This is called the chemical shift. Hence, one can see if, for instance, silicon is in oxidation state 0 (Si-Si bond in bulk silicon) or oxidation state +IV (Four Si-0 bonds in SiO2) by looking at the binding energy of the core level electrons.
The figure to the left illustrates the chemical shift. Si2p XPS spectra were recorded an a Si reference sample and a Si sample that was treated in HF prior to the measurement - and the spectra are clearly different. The spectrum of untreated Si has a clear feature at about 103 eV due to Si atoms bonded to oxygen in the native oxide. In the spectrum from the HF treated sample, only the feature arising from Si-Si interaction is present. Note that both curves only shows the Si signal, but with an clear indication of the chemical state of the Si atoms in the samples.
If you study polymers, you can detect the presence of different chemical groups, for example (C-C),(C-OH),(C=O),(CF3) or (CF2-CH2) in the polymeric layer. And after surface treatments, you may examine differences in the polymeric layer.
Note that binding energies for different chemical states often can be found in the literature.
