Specific Process Knowledge/Thin Film deposition/ALD/Al2O3 deposition using ALD: Difference between revisions
Created page with " =<span style="background:#FF2800">THIS PAGE IS UNDER CONSTRUCTION</span>200px=" |
No edit summary |
||
| (23 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
=< | '''Feedback to this page''': '''[mailto:labadviser@nanolab.dtu.dk?Subject=Feed%20back%20from%20page%20http://labadviser.nanolab.dtu.dk/index.php/Specific_Process_Knowledge/Thin_Film_deposition/ALD/Al2O3_deposition_using_ALD click here]''' | ||
''This page is written by DTU Nanolab internal'' | |||
The ALD window for depostion of aluminium oxide (Al<sub>2</sub>O<sub>3</sub>) ranges from 150 <sup>o</sup>C to 300 <sup>o</sup>C. XPS measurements shows that at temperatures below 150 <sup>o</sup>C the Al<sub>2</sub>O<sub>3</sub> layer will be contaminated by unreacted TMA molecules, and at temperatures above 300 <sup>o</sup>C the TMA decomposes. | |||
All results shown on this page have been obtained by depositing Al2O3 on new Si(100) wafers with native oxide using the "AL2O3" recipe. | |||
====Al<sub>2</sub>O<sub>3</sub> standard recipe==== | |||
<b>Recipe</b>: AL2O3 | |||
<b>Temperature</b>: 150 <sup>o</sup>C - 350 <sup>o</sup>C | |||
{| border="2" cellspacing="2" cellpadding="5" align="none" | |||
|- | |||
| | |||
!TMA | |||
!H<sub>2</sub>O | |||
|- | |||
!Nitrogen flow | |||
|150 sccm | |||
|200 sccm | |||
|- | |||
!Pulse time | |||
|0.1 s | |||
|0.1 s | |||
|- | |||
!Purge time | |||
|3.0 s | |||
|4.0 s | |||
|- | |||
|} | |||
====Al<sub>2</sub>O<sub>3</sub> deposition rates==== | |||
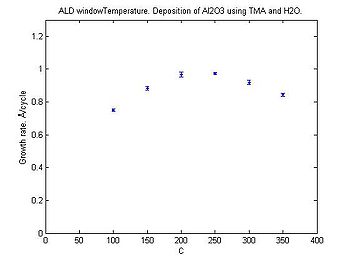
The deposition rate for Al<sub>2</sub>O<sub>3</sub> depends on the temperature, see the graph below. | |||
{| border="1" cellspacing="1" cellpadding="2" | |||
! | |||
[[Image:ALD window Al2O3 H2O.jpg|350x350px|thumb|center|Al<sub>2</sub>O<sub>3</sub> deposition rate as function of temperature.]] | |||
|} | |||
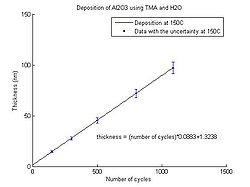
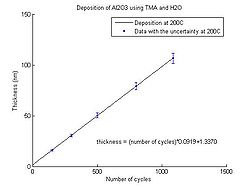
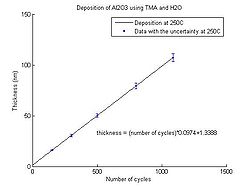
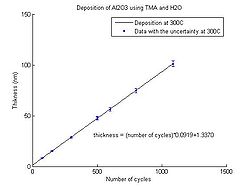
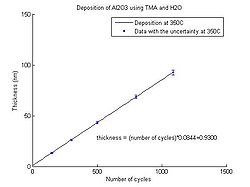
In the graphs below the Al<sub>2</sub>O<sub>3</sub> thickness as function of number of cycles for deposition temperatures between 150 <sup>o</sup>C and 350 <sup>o</sup>C can be seen. The points have been fitted to a linear curve, and the slope of this curve shows the deposition rate. So from the equation the number of cycles required for a certain thickness of Al<sub>2</sub>O<sub>3</sub> to be deposited can be calculated. | |||
<gallery caption="Aluminium oxide thickness as function of number of cycles" widths="250px" heights="250px" perrow="5"> | |||
image:ALD Al2O3 grow rate 150C.jpg| Temperature 150 <sup>o</sup>C. | |||
image:ALD Al2O3 grow rate 200C.jpg| Temperature 200 <sup>o</sup>C. | |||
image:ALD Al2O3 grow rate 250C.jpg| Temperature 250 <sup>o</sup>C. | |||
image:ALD Al2O3 grow rate 300C.jpg| Temperature 300 <sup>o</sup>C. | |||
image:ALD Al2O3 grow rate 350C.jpg| Temperature 350 <sup>o</sup>C. | |||
</gallery> | |||
Evgeniy Shkondin, DTU Nanolab, February-March 2014. | |||
Latest revision as of 10:26, 19 June 2023
Feedback to this page: click here
This page is written by DTU Nanolab internal
The ALD window for depostion of aluminium oxide (Al2O3) ranges from 150 oC to 300 oC. XPS measurements shows that at temperatures below 150 oC the Al2O3 layer will be contaminated by unreacted TMA molecules, and at temperatures above 300 oC the TMA decomposes.
All results shown on this page have been obtained by depositing Al2O3 on new Si(100) wafers with native oxide using the "AL2O3" recipe.
Al2O3 standard recipe
Recipe: AL2O3
Temperature: 150 oC - 350 oC
| TMA | H2O | |
|---|---|---|
| Nitrogen flow | 150 sccm | 200 sccm |
| Pulse time | 0.1 s | 0.1 s |
| Purge time | 3.0 s | 4.0 s |
Al2O3 deposition rates
The deposition rate for Al2O3 depends on the temperature, see the graph below.
 |
|---|
In the graphs below the Al2O3 thickness as function of number of cycles for deposition temperatures between 150 oC and 350 oC can be seen. The points have been fitted to a linear curve, and the slope of this curve shows the deposition rate. So from the equation the number of cycles required for a certain thickness of Al2O3 to be deposited can be calculated.
- Aluminium oxide thickness as function of number of cycles
-
Temperature 150 oC.
-
Temperature 200 oC.
-
Temperature 250 oC.
-
Temperature 300 oC.
-
Temperature 350 oC.
Evgeniy Shkondin, DTU Nanolab, February-March 2014.