Specific Process Knowledge/Etch/Aluminum Oxide/Al2O3 Etch using HF: Difference between revisions
No edit summary |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
'''Feedback to this page''': '''[mailto:labadviser@danchip.dtu.dk?Subject=Feed%20back%20from%20page%20http://labadviser.nanolab.dtu.dk/index.php/Specific_Process_Knowledge/Aluminum_Oxide/Al2O3_Etch_using_HF click here]''' | '''Unless anything else is stated, everything on this page, text and pictures are made by DTU Nanolab.''' | ||
'''All links to Kemibrug (SDS) and Labmanager Including APV and QC requires login.''' | |||
'''Feedback to this page''': '''[mailto:labadviser@danchip.dtu.dk?Subject=Feed%20back%20from%20page%20http://labadviser.nanolab.dtu.dk/index.php/Specific_Process_Knowledge/Etch/Aluminum_Oxide/Al2O3_Etch_using_HF click here]''' | |||
| Line 6: | Line 10: | ||
=== Experiment and results === | === Experiment and results === | ||
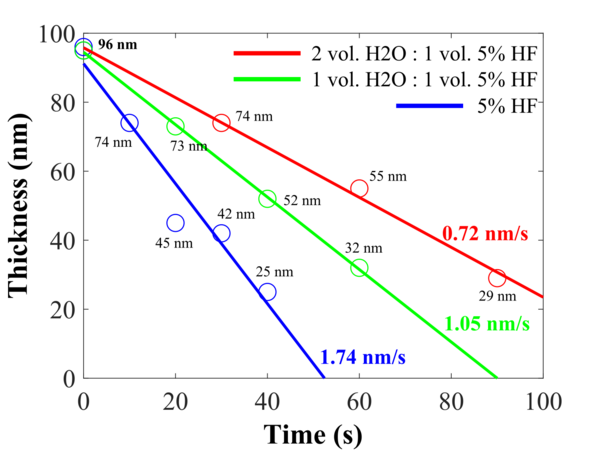
Si samples with about 100 nm of [[Specific_Process_Knowledge/Thin_film_deposition/ALD_Picosun_R200|ALD]] deposited Al<sub>2</sub>O<sub>3</sub> (1000 cycles at 300<sup>o</sup>C) has been etched in different HF concentrations. After the etching, the thickness of the Al<sub>2</sub>O<sub>3</sub> layer has been measured, and the thickness as function of time has been plotted as shown in the graph below. | Si samples with about 100 nm of [[Specific_Process_Knowledge/Thin_film_deposition/ALD_Picosun_R200|ALD]] deposited Al<sub>2</sub>O<sub>3</sub> (1000 cycles at 300<sup>o</sup>C) has been etched in different HF concentrations. After the etching, the thickness of the Al<sub>2</sub>O<sub>3</sub> layer has been measured using [[Specific_Process_Knowledge/Characterization/Optical_characterization#Ellipsometer|Ellipsometer VASE]], and the thickness as function of time has been plotted as shown in the graph below. | ||
<gallery caption="" widths="600px" heights="500px" perrow="1"> | <gallery caption="" widths="600px" heights="500px" perrow="1"> | ||
| Line 15: | Line 19: | ||
The etch rates: | The etch rates: | ||
<ul> | <ul> | ||
<li> <p>5% HF → <b>1.74 nm/s</b> </p></li> | <li><p>5% HF → <b>1.74 nm/s</b></p></li> | ||
<li> 1vol. H<sub>2</sub>O : 1vol. 5% HF <b>1.05 nm/s</b> </li> | <li><p> 1vol. H<sub>2</sub>O : 1vol. 5% HF → <b>1.05 nm/s</b></p></li> | ||
<li> 2vol. H<sub>2</sub>O : 1vol. 5% HF <b>0.72 nm/s</b> </li> | <li><p> 2vol. H<sub>2</sub>O : 1vol. 5% HF → <b>0.72 nm/s</b></p></li> | ||
</ul> | </ul> | ||
Latest revision as of 15:37, 6 February 2023
Unless anything else is stated, everything on this page, text and pictures are made by DTU Nanolab.
All links to Kemibrug (SDS) and Labmanager Including APV and QC requires login.
Feedback to this page: click here
A wet chemical etch of Al2O3 can be done with HF. The etch rate depends on the HF concentration.
Experiment and results
Si samples with about 100 nm of ALD deposited Al2O3 (1000 cycles at 300oC) has been etched in different HF concentrations. After the etching, the thickness of the Al2O3 layer has been measured using Ellipsometer VASE, and the thickness as function of time has been plotted as shown in the graph below.
-
Different etching rates for different HF concentrations.
The etch rates:
5% HF → 1.74 nm/s
1vol. H2O : 1vol. 5% HF → 1.05 nm/s
2vol. H2O : 1vol. 5% HF → 0.72 nm/s
Be aware of that the 5% HF etches quite fast. Actually, so fast that it can be tricky to control. Taking the sample out of the solution and placing it in a bigger water container to rinse, resulting a time delay due to the movement and the etch continues. It means the handling things around the fumehood can be a source of errors in this case.
Evgeniy Shkondin, DTU Nanolab, June 2019