LabAdviser/314/Microscopy 314-307/Technique/Holo/Off-axis ATEM
< LabAdviser | 314 | Microscopy 314-307 | Technique/Holo
Jump to navigation
Jump to search
Feedback to this page: click here
(content by Ganapathi Prabhu Sai Balasubramanian @DTU Nanolab, April 2020)
Procedure for Performing Electron Holography in the ATEM
Setting up the ATEM for holography
- Fill Liquid nitrogen in the dewar twice or thrice; once at the beginning of the session prior to inserting the TEM holder, once after 30 minutes into the session, and the once around half time of the full-day session.
- Switch on the power supply at the backside of the room
-
- Switch on the control box (under the microscope PC screen)
-
- Switch cables on SAD from red to grey
- Load the appropriate TEM alignment, e.g. 120 keV TEM. This is done by going to Alignment tab → Alignment → Flapout → load the alignment file for the correct keV, e.g. Main_120kV. Select one particular alignment and the press Ctrl+A for selecting all and then move them from “Available” to “Selected” by clicking on the Apply button.
- Load the FEG register for TEM for the electron energy chosen in step 5 above. This is done by going to Gun tab → FEG Register → Choose the correct register → Click on Set
- If there is a sample holder already in the microscope, reset the holder and take it out. Then insert your sample holder. Insert the sample holder with the pin positioned at 5 o’clock and go straight and as far as possible and stop. The color of the turbo on button should go from grey to orange and then to yellow. Look for the airlock cycle to start and if it doesn’t start, then rotate the holder a bit to make it start. Once it has started, wait for it to complete, it should take 10 minutes to complete. Then rotate the holder anti-clockwise from the 5 o’clock to 12 o’clock position and then insert the holder rest of the way straight and until it stops.
- Make sure that the spot size is 3. You can read from the bottom panel of the microscope PC what the spot size is and then play with R3 button on the right control panel for increasing the spot size and with L3 button on the left control panel for decreasing the spot size.
- Make sure that the size of C2 aperture is set to 150 µm. Go to Mono tab → Aperture → C2 → adjust and play with multifunctional knobs MFx and MFy on the left and right control panels for centering the chosen C2 aperture.
- Turn column valve open. For doing this click on the yellow colored column valve, upon opening, its color will go from yellow to grey.
- Find the sample. Then, go to Stage tab → Click on tracks button. This will track your positions on sample. Go to a low enough magnification in the SA range and go around the sample and find the feature of interest.
- Acquire an image of the feature of interest.
- While in the TEM mode go to a low magnification e.g. 30,000. Go to Mono tabMonochromator panel Click on the Shift button and play with MFx and MFy for getting the the beam centered (?).
- Go to Mono tab → Monochromator panel → Click on Focus button and play with the intensity knob, adjust the beam size with the intensity knob so that the monochromator edges just disappear. We want a nice round beam, so we want the beam with the rough perimeter to be limited by the C2 aperture. The focus value should be -40 to -30. Turn off the Shift and the Focus buttons.
- Turn C3 off
- For doing this go to Gun tab → Free Ctrl → C3 off
- For doing this go to Gun tab → Free Ctrl → C3 off
- Go to Holography tab → Click on Holography button
- Go to Lorentz → Click on Lorentz button
- Don’t try to see the sample at this stage. The bottom panel of the microscope PC will read a magnification something in the Ltz SA range (e.g. Ltz SA 3800X). We want a magnification in the Mh mode. By changing the magnification with the magnification knob go to Ltz Mh 520X. Find the beam. Normally you see it. If you don’t see it first, play with the intensity knob and then the magnification, go to a lower magnification.
- Don’t try to see the sample at this stage. The bottom panel of the microscope PC will read a magnification something in the Ltz SA range (e.g. Ltz SA 3800X). We want a magnification in the Mh mode. By changing the magnification with the magnification knob go to Ltz Mh 520X. Find the beam. Normally you see it. If you don’t see it first, play with the intensity knob and then the magnification, go to a lower magnification.
- Go to Holography tab → Direct Alignment. Click on Beam Shift and center the beam with multifunctional knobs MFx and MFy. Don’t park the beam on the sample after you find the beam.
- Choose a good magnification and work only at that magnification. A magnification of 53000X in LtzMh works for the GaAs nanowires.
- If the sample is slightly out of focus then don’t try to fix it. Also, if the FFT of the image shows signs of astigmatism, then don’t try to fix it.
- If the sample is slightly out of focus then don’t try to fix it. Also, if the FFT of the image shows signs of astigmatism, then don’t try to fix it.
- Set the biprism. Insert the SAD aperture (lever from right to left). Position of the biprism is SAD aperture #1. Find the biprism and center it. If you can’t see it, excite the biprism to ~100 V with the volume knob on the controls for the biprism.
-
- Align the long axis of the biprism wire in such a way that it is parallel to the long axis of the feature of interest in case of nanowire in plan view OR align the long axis of the biprism wire along the edge of the lamella parallel to the grid, in case of dealing with specimens in the form of a lamella. For rotating the biprism wire, turn the black cylinder attached to the area where the controls for the SAD aperture exist in the microscope. Next, play with condenser stigmatism controls for getting the beam elliptical. When the beam is condensed it should be elliptical with the long axis of the ellipse perpendicular to the biprism wire. Upon turning the intensity knob clockwise, when the beam expands, the long axis of the ellipse should be parallel to the biprism wire. If what you see is opposite of this then you should try swapping the MFx and MFy values.
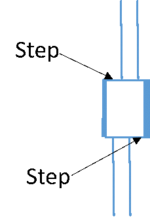
- Note that either MFx or MFy has to be +1 or -1 the other one can be anything including +1 and -1. After the MFx and MFy are set to the correct values, you should have an elliptical beam, that looks as described above. Additionally, you should not see the steps in the beam (check this at a high enough mag, say Ltz Mh 18000X; but not too high as there won’t be enough signals) when it is elliptical and condensed and when the potential across the birpism is at least 50 V (see the figure on left). To correct this step, play with that MF control that was not kept constant at +1 or -1 while the elliptical beam was rotated to make it perpendicular to the biprism wire, when the beam is condensed.
-
- Find the region of interest and do alignments-beam shift and rotation centering. Go to Holography tab → Direct Alignments → Beam shift and play with MFx and MFy to get the beam to the center. Next, Holography tab → Direct Alignments → rotation centering and play with MFx and MFy to get the image of the region of interest to go in and out of focus without any lateral shift when the rotation centering under Direct Alignments is activated.
- In the monitor for the camera PC, go to Digital Micrograph software → camera tab → camera → HREMCCD Camera. Make sure this camera is selected. Then insert the camera. Then start view of the camera and lift the screen in the microscope (click on R1 button). Make sure that you are at a magnification wherein you can see the fringes across the diameter of the region of interest. If there is any defect on the hologram that may be due to the biprism, play with the controls for the SAD aperture to move the biprism and therefore the defect in the plane of the camera and out of the FOV. Condense the beam with the intensity knob and center it with the track ball so that the FOV without the region of interest is homogeneously illuminated.
- Correct for objective lens astigmatism. For every magnification change you should recheck objective lens astigmatism and the focus (with the focus knob). You do the rotation centering at the beginning and not when you play with the magnification-control.
- Acquire a reference hologram with acquisition time of 10 s and if it is noisy reacquire the hologram with 10 s or 5 s. Then draw a spectrum line across a few fringes and see how many pixels are there per fringe. We should keep 4 pixels per fringe. To increase the number of fringes per unit are, increase the biprism voltage. A biprism voltage of 120-130V is recommended.
Finishing-up the holography part of the measurement
- Stop the camera acquisition
- Put the viewing screen down (with R1 button)
- Come out of the Lorentz and Holography modes. To do this go to Holography tab → Click on Holography button again to unselect it. Then go to Lorentz → Click on Lorentz button again to unselect it. Then switch to the TEM mode (Gun → BeamSettings → Click on TEM button).
- Turn down the volume knob of the biprism. Then turn off this box.
- Turn off the power supply box in the back of the room.
- Swap the cables at the SAD apertures (from grey to red)
- Switch to aperture #3. Remove the SAD apertures from the column by moving the lever from left to right.
Acquiring CBED patterns
- Decrease the magnification to SA 7200X or something in the SA range.
- Start the camera acquisition. Lift the viewing screen (press R1 button)
- Go to Mono tab → Monochromator tune → Click on focus button and play with the intensity knob by turning it clockwise and decrease the intensity drastically, say to the value of 100 or 175 or 200, the higher the better as there will be less damage to the sample. We want to have a beam that gives a CBED pattern that is not saturated under acquisition times of 0.001-0.01 s. This way you will get a beam that doesn’t burn the featyre of interest. Go to a magnification around SA 49000X.
- Go to Gun tab → Click on the TEM button
- Acquire CBED pattern after tilting the sample appropriately. Use an acquisition time of 0.001 sec or 0.01 sec. Use an acquisition time as low as possible otherwise you will burn the camera.
Closing down the session
- Stop the camera acquisition.
- Put the screen down (press R1 button)
- Reset the holder
- Make sure that all apertures (SAD and objective) are retracted.
- Close the column isolation valve. Go to Set up tab → Vacuum → Click on column valve closed button (the button’s color will change from grey to yellow)
- Remove the holder
- Fill out the process log
- Check on the lab manager whether there is a next user
For the next user: Changing the beam kV and settings for the alignment and FEG register
- Go to Set up → High Tension → Decrease/Increase the HT in steps from 300 kV/120 kV to the needed setting
- Go to Alignments tab → Alignments → Flapout → select the required setting (e.g. Main_120kV) and then press Ctrl+A for selecting all and then move them from “Available” to “Selected” by clicking on the Apply button.
- Go to Gun → FEG Register → Load the FEG register for the electron energy of interest to the next user
- Go to Setup tab → Vacuum → Flapout → Click on the cryo cycle button and it will turn yellow
- Stop your session in Lab Manager
- Log out from lab manager and the support PC.
Notes/Tips
- For obtaining electron holograms from the region of interest, tilt the sample appropriately so that the diffraction contrast within the region of interest i.e. black areas are at a minimum
- Avoid Fresnel fringe in the camera’s field of view, so center the biprism wire accordingly
- In case of a core-shell structure, go to a high enough biprism voltage so that you can see the edges of the core sharp.
- While obtaining electron holograms from the sample you must be away from the membrane edges; you should have only the feature of interest in the holographic field of view
- The fringes in the object and reference holograms must be sharp, if they aren’t sharp reacquire them
- In obtaining reference holograms be sure to keep away from all parts of the sample; you should have only vacuum in the reference holograms.
- In the object holograms, put the sample at focus using the focus knob; the object edges will be sharp when it is at focus. If there is a hole near the object of interest then it will be sharp when in focus. You can use the edges of the hole for the correction of objective lens astigmatism. The fringe contrast around the holde should be homogeneous.
- The width of the feature of interest should be smaller than the fringe width of the biprism.
- The holographic FOV should be at the center of the image field-of-view
- Obtain many, say, 5-10 sets of the reference and object holograms for each condition
- Do the rotation centering before adjusting objective focus
- Remember that in Lorentz mode we can’t get a diffraction pattern
- You should aim to have 4-5 steps per fringe in the reference and object holograms.
- For analyzing the holograms while the experiment is running, in Digital Micrograph, go to
- Holo3 → Reconstruct → Reconstruct with ref, OR
- Holo3 → Reconstruct → Reconstruc. (reconstruct using just one image) OR
- Holo3 → Reconstruct → Reconstruct with ++, press space bar and adjust the mask size with the up and down arrow keys, press space bar and this will give you the phase image
- Go to a high enough biprism voltage to have fine enough fringes that will lead to non-overlapping central and side bands in the FFT
- Make sure that the fringes contrast across the imaging FOV is uniform.
- If in the imaging FOV of the reference fringes, you see some black areas then try spreading the beam. If you see some disturbance (e.g. curving) in the fringes of the reference it could be due to either nearness of the sample or some charged contaminants on the birism. So to get rid of it, move the sample away and/or move to a different part of the biprism my moving along its length.
- When obtaining CBED patterns ensure that you can clearly see features in the central disk
- If during your experiment involving electron holography, you see that the visibility of fringes has gone low, then for increasing the visibility of the fringes you can decrease the exposure time, decrease the biprism voltage, reduce the magnification, play with track ball and the intensity knob to get the beam to uniformly illuminate the region of interest.