LabAdviser/314/Preparation 314-307/Soft-matter
Feedback to this page: click here
Plunge Freezer
Leica EM GP2
The plunge freezing is a cryo-fixation method used to preserve samples in their most native state prior to cryo-electron microscopy.
The main steps of this technique proceed as follows:
1. The sample is spread into a thin film across an EM grid
2. The liquid droplet is then blotted with filter paper until only a very thin film of fluid remains
3. The grid is then rapidly plunged into a cryogen (usually liquid ethane)
4. The grid is afterwards stored in a grid box submerged in liquid nitrogen until finally loaded into the cryo-electron microscope for imaging
Operating manual: File:Leica EM GP2 Operating manual.pdf
Risk Assessment: File:Risk assessment - EM GP2 Plunge Freezer.pdf

For further information on the equipment usage or training contact mktracy@dtu.dk [1].
Microtomes
RMC MT-7 Microtome
Our RMC MT-7 Microtome is a vintage piece of equipment and can be used for planning specimens for SEM/EDX and/or for cutting relative thin slices (approx. 200nm thick) for TEM. First, the specimen needs to be embedded into a resin or an epoxy, and then can be sliced with a glass or diamond knife.
Risk Assessment of RMC MT-7 Microtome: File:APV RMC Microtome.pdf

For further information about the microtome usage contact afull@dtu.dk [2].
Leica EM UC7 Ultramicrotome
The Leica ultramicrotome is used for ultra thin sectioning of sample embedded in resin block with a feed range of 1 nm up to 15 µm. Glass or diamond knife is used for the ultra sectioning of the samples.
The main steps of this technique proceed as follows:
1. Trim the resin embedded sample
2. Mount sample
3. Mount glass or diamond knife to knife holder
4. Ultrasectioning of sample
5. Fishing sections
6. Transfer to an EM grid
Operating manual: File:EM UC7 Operating manual.pdf
Risk Assessment: File:Risk assessment - EM UC7 Ultramicrotome.pdf

For further information about the equipment usage or training contact mktracy@dtu.dk [3].
Leica EM FC7 Cryo-Ultramicrotome
Within minutes the Leica EM UC7 ultramicrotome can change to a cryo-ultramicrotome by mounting the cryo chamber EM FC7. Cryo-sections (-15° to -185°C) can be prepared for TEM, SEM, AFM and LM.
The main steps of this technique proceed as follows:
1. Set up the cryo chamber EM FC7 to the EM UC7 apparatus
2. Mount both the trim and sectioning diamond knives to the knive holder and set up in the cryo chamber
3. Fill up liquid nitrogen Dewar
4. Set pump between the liquid nitrogen Dewar and the ultramicrotome apparatus
5. Let equipment to cool down for 1 hour
6. Meanwhile prepare samples
7. Mount sample
8. Trimming and ultrasectioning of sample
9. Collecting sections and transfer to an EM grid
Operating manual: File:EM FC7 Operating manual.pdf
Risk Assessment: File:Risk assessment - EM FC7 Cryo-Ultramicrotome.pdf
For further information about the equipment usage or training contact mktracy@dtu.dk [4].

LKB Knifemaker 7801A
At DTU Nanolab we offer the possibility to make glass knives using the LKB Knifemaker 7801A.
Manual of LKB Knifemaker 7801A: File:LKB Knifemaker 7800B Manual.pdf
For further information about the knifemaker usage and training contact mktracy@dtu.dk [5].

Critical point dryer
Leica EM CPD300
The Critical Point Drier (CPD) is used for drying samples for the SEM. The machine uses the fact that at the critical point the solvent can be converted from liquid to gas without crossing the interfaces between liquid and gas (no surface tension) and hence no sample drying artefacts. CO2 is the solvent of choice as its triple point is suitable for biological samples (31°C and 74 bars). The CO2 is not miscible with water; therefore the sample should be dissolved in acetone or ethanol.
Operating manual: File:EM CPD300 Operating manual.pdf
Risk Assessment: File:Risk assessment - EM CPD300 Critical Point Dryer.pdf
For further information on the CPD usage or training contact mktracy@dtu.dk [6].

Centrifuge
The Mini Spinner Eppendorf is a desktop centrifuge with supplied rotor with maximum speed/force 13,400rpm/12,100xG. It has a 30-minute timer, which is settable in 15-sec increments. It has a short-spin mode fixed at maximum speed. The Mini Spinner Eppendorf is located in building 314, in the Soft Mater (toxic) Lab.
For further information on the equipment usage contact mktracy@dtu.dk [7].

pH meter
If you are planning on doing chemical fixation on biological samples you will probably need to make buffers and use a pH-meter. The pH-meter (model pH 1000L) is located in building 314, in the Soft Mater (toxic) Lab and it is dedicated to “Soft Matter” related work.
For further information on the equipment usage contact mktracy@dtu.dk [8].

TS9026 Drying oven
The drying oven is of 26L capacity and is used for epoxy experiments.
For further information on the equipment usage contact mktracy@dtu.dk [9].

Mini tube rotator
The mini-tube rotator is designed for rotating various sized laboratory tubes at a variety of mixing angles at speeds ranging from 4 to 18 min–1. Ideal for mixing and separation processes involved in molecular biology, histochemistry and clinical applications.
- Variable speed with LCD display
- Digital microprocessor control
- An adjustable 0 – 90° mixing angle
- Interchangeable carousels for all common laboratory tubes
- Cold room and incubator compatible
For further information on the equipment usage contact mktracy@dtu.dk [10].

Pulsing vortex mixer
Pulsing vortex mixer with features to suit different applications. The vortex mixer provide optimum comfort and minimal stress.
- Can be used at 4 to 40 °C (maximum 85% relative humidity, non condensing), in an incubator, CO₂ incubator or cold room
- Pulsing model has unique action which reduces heat generation and ensures efficient mixing and disruption
- Digital and pulsing units have timer, 1 s to 160 h
For further information on the equipment usage contact mktracy@dtu.dk [11].

Sample preparation flowchart
For TEM imaging of biological sample at room temperature or cryo conditions:
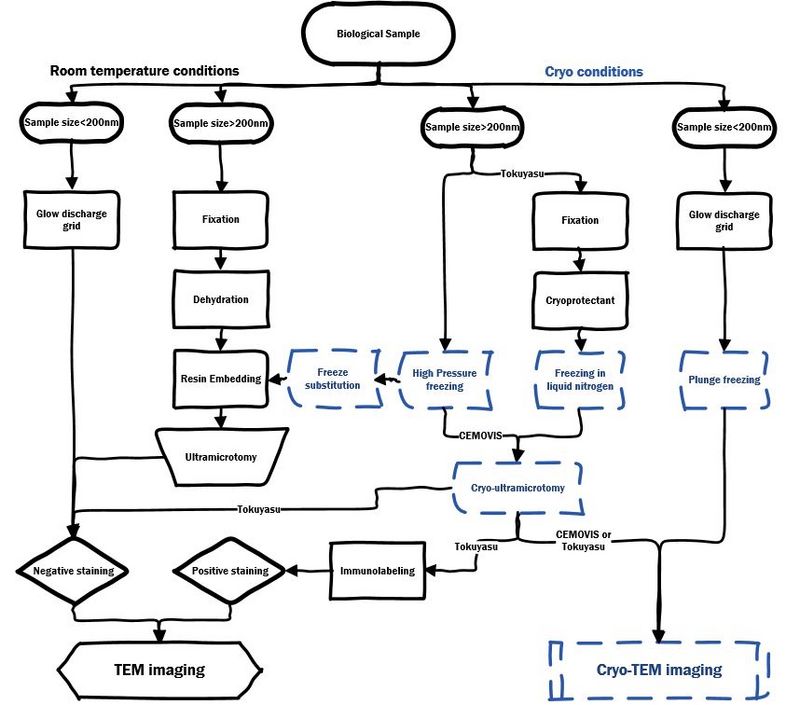
For SEM imaging of biological sample at room temperature or cryo conditions:

For further information about the procedures contact mktracy@dtu.dk [12].
